Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation
- PMID: 10899880
- PMCID: PMC98424
- DOI: 10.1128/IAI.68.8.4736-4745.2000
Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation
Abstract
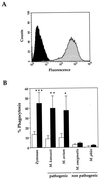
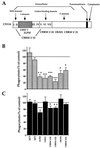
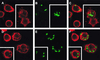
Complement receptor type 3 (CR3) was initially described as an opsonic receptor. Subsequently, CR3-mediated lectin-sugar recognition mechanisms have been shown to play a major role in the nonopsonic phagocytosis of several pathogens, among them Mycobacterium tuberculosis. Little is known about the binding and signal transduction mechanisms operating during nonopsonic ingestion through CR3 of different microorganisms. In the present study, we used CHO cells stably transfected with CR3 to show that CR3 was able to mediate internalization of zymosan and pathogenic mycobacteria (Mycobacterium kansasii and Mycobacterium avium) but not that of nonpathogenic species (Mycobacterium smegmatis and Mycobacterium phlei). A combination of mannan and beta-glucan inhibited the phagocytosis of zymosan but had no effect on M. kansasii ingestion. Among six monoclonal antibodies (MAbs) directed against the CD11b subunit of CR3 that decreased zymosan ingestion, only three inhibited M. kansasii phagocytosis. In particular, MAbs known to block the CR3 lectin site affected only internalization of zymosan. Using U937 macrophages, we observed that zymosan ingestion through CR3 induced superoxide production measured by cytochrome c reduction and by translocation of the NADPH oxidase cytosolic component p47phox to the phagosomal membrane, whereas phagocytosis of viable or heat-killed M. kansasii did not. Furthermore, lack of superoxide anion production during phagocytosis of M. kansasii was not due to inhibition of NADPH oxidase per se or superoxide anion scavenging. Together, our results indicate that (i) nonopsonic phagocytosis of zymosan and M. kansasii by CR3 implicates different molecular mechanisms involving multiple and distinct epitopes of CD11b and (ii) CR3 may transduce different cellular responses depending on the sites mediating nonopsonic phagocytosis.
Figures



References
-
- Balsam L B, Liang T W, Parkos C A. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol. 1998;160:5058–5065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

