Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction
- PMID: 10899903
- PMCID: PMC2193256
- DOI: 10.1084/jem.192.2.159
Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction
Abstract
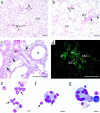
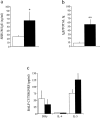
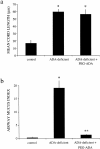
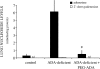
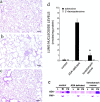
Adenosine deaminase (ADA) is a purine catabolic enzyme that manages levels of the biologically active purines adenosine and 2'-deoxyadenosine in tissues and cells. ADA-deficient mice die at 3 wk of age from severe respiratory distress. This phenotype is progressive and is linked to perturbations in pulmonary purine metabolism. The inflammatory changes found in the lungs of ADA-deficient mice included an accumulation of activated alveolar macrophages and eosinophils. These changes were accompanied by a pronounced enlargement of alveolar spaces and increases in mucus production in the bronchial airways. The alveolar enlargement was found to be due in part to abnormal alveogenesis. Lowering adenosine and 2'-deoxyadenosine levels using ADA enzyme therapy decreased the pulmonary eosinophilia and resolved many of the lung histopathologies. In addition, genetically restoring ADA to the forestomach of otherwise ADA-deficient mice prevented adenine metabolic disturbances as well as lung inflammation and damage. These data suggest that disturbances in purinergic signaling mediate the lung inflammation and damage seen in ADA-deficient mice.
Figures



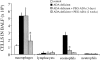
References
-
- Frederiksen S. Specificity of adenosine deaminase toward adenosine and 2′-deoxyadenosine analogues. Arch. Biochem. Biophys. 1966;113:383–388. - PubMed
-
- Olah M.E., Stiles G.L. Adenosine receptor subtypescharacterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–606. - PubMed
-
- Benveniste P., Zhu W., Cohen A. Interference with thymocyte differentiation by an inhibitor of S-adenosylhomocysteine hydrolase. J. Immunol. 1995;155:536–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

