Interactions between abscisic acid and ethylene signaling cascades
- PMID: 10899977
- PMCID: PMC149052
- DOI: 10.1105/tpc.12.7.1103
Interactions between abscisic acid and ethylene signaling cascades
Abstract
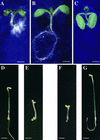
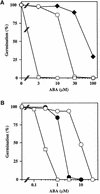
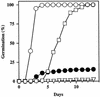
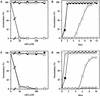
We screened for mutations that either enhanced or suppressed the abscisic acid (ABA)-resistant seed germination phenotype of the Arabidopsis abi1-1 mutant. Alleles of the constitutive ethylene response mutant ctr1 and ethylene-insensitive mutant ein2 were recovered as enhancer and suppressor mutations, respectively. Using these and other ethylene response mutants, we showed that the ethylene signaling cascade defined by the ETR1, CTR1, and EIN2 genes inhibits ABA signaling in seeds. Furthermore, epistasis analysis between ethylene- and ABA-insensitive mutations indicated that endogenous ethylene promotes seed germination by decreasing sensitivity to endogenous ABA. In marked contrast to the situation in seeds, ein2 and etr1-1 roots were resistant to both ABA and ethylene. Our data indicate that ABA inhibition of root growth requires a functional ethylene signaling cascade, although this inhibition is apparently not mediated by an increase in ethylene biosynthesis. These results are discussed in the context of the other hormonal regulations controlling seed germination and root growth.
Figures





References
-
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press).
-
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. - PubMed
-
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. - PubMed
-
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1089. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

