Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis
- PMID: 10899978
- PMCID: PMC149053
- DOI: 10.1105/tpc.12.7.1117
Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis
Abstract
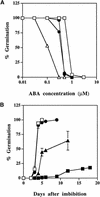
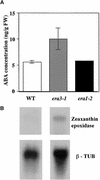
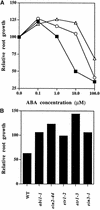
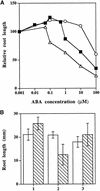
Although abscisic acid (ABA) is involved in a variety of plant growth and developmental processes, few genes that actually regulate the transduction of the ABA signal into a cellular response have been identified. In an attempt to determine negative regulators of ABA signaling, we identified mutants, designated enhanced response to ABA3 (era3), that increased the sensitivity of the seed to ABA. Biochemical and molecular analyses demonstrated that era3 mutants overaccumulate ABA, suggesting that era3 is a negative regulator of ABA synthesis. Subsequent genetic analysis of era3 alleles, however, showed that these are new alleles at the ETHYLENE INSENSITIVE2 locus. Other mutants defective in their response to ethylene also showed altered ABA sensitivity; from these results, we conclude that ethylene appears to be a negative regulator of ABA action during germination. In contrast, the ethylene response pathway positively regulates some aspects of ABA action that involve root growth in the absence of ethylene. We discuss the response of plants to ethylene and ABA in the context of how these two hormones could influence the same growth responses.
Figures



References
-
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press).
-
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. - PubMed
-
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. - PubMed
-
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

