The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants
- PMID: 10899983
- PMCID: PMC149058
- DOI: 10.1105/tpc.12.7.1179
The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants
Abstract
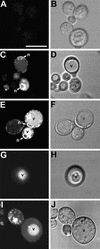
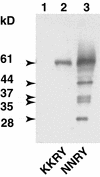
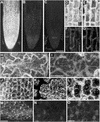
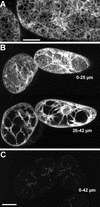
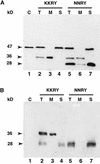
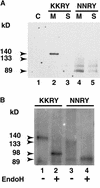
The tomato Cf-9 disease resistance gene encodes a type I membrane protein carrying a cytosolic dilysine motif. In mammals and yeast, this motif promotes the retrieval of type I membrane proteins from the Golgi apparatus to the endoplasmic reticulum (ER). To test whether the C-terminal KKXX signal of Cf-9 is functional as a retrieval motif and to investigate its role in plants, green fluorescent protein (GFP) was fused to the transmembrane domain of Cf-9 and expressed in yeast, Arabidopsis, and tobacco cells. The fusion protein was targeted to the ER in each of these expression systems, and mutation of the KKXX motif to NNXX led to secretion of the fusion protein. In yeast, the mutant protein reached the vacuole, but plants secreted it as a soluble protein after proteolytic removal of the transmembrane domain. Triple hemagglutinin (HA)-tagged full-length Cf-9 was also targeted to the ER in tobacco cells, and cleavage was also observed for the NNXX mutant protein, suggesting an endoprotease recognition site located within the Cf-9 lumenal sequence common to both the GFP- and the HA-tagged fusions. Our results indicate that the KKXX motif confers ER localization in plants as well as mammals and yeast and that Cf-9 is a resident protein of the ER.
Figures







References
-
- Andersson, H., Kappeler, F., and Hauri, H.-P. (1999). Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem. 274, 15080–15084. - PubMed
-
- Andreeva, A.V., Kutuzov, M.A., Evans, D.E., and Hawes, C.R. (1998). Proteins involved in membrane transport between the ER and the Golgi apparatus: 21 putative plant homologues revealed by dbEST searching. Cell. Biol. Int. 22, 145–160. - PubMed
-
- Andres, D.A., Rhodes, J.D., Meisel, R.L., and Dixon, J.E. (1991). Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J. Biol. Chem. 266, 14277–14282. - PubMed
-
- Arondel, V., Lemieux, B., Hwang, I., Gibson, S., Goodman, H.M., and Somerville, C.R. (1992). Map-based cloning of a gene controlling omega-3 fatty acid desaturation in Arabidopsis. Science 258, 1353–1355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

