Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (sst2) gene expression and administration of cytotoxic somatostatin analog AN-238
- PMID: 10900262
- PMCID: PMC16842
- DOI: 10.1073/pnas.130196697
Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (sst2) gene expression and administration of cytotoxic somatostatin analog AN-238
Abstract
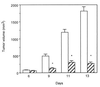
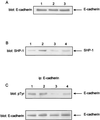
The sst2 somatostatin receptor mediates the antiproliferative effects of somatostatin analogs. The present study demonstrates that stable expression of sst2 in the hamster pancreatic cancer cells PC-1 and PC-1.0 activates an autocrine negative loop leading to an in vitro inhibition of cell proliferation. In vivo studies conducted in Syrian golden hamsters after orthotopic implantation of PC-1.0 cells showed that both tumor growth and metastatic progression of allografts containing 100% of sst2-expressing cells were significantly inhibited for up to 20 days after implantation, as compared with control allografts that did not express sst2. A local antitumor bystander effect was observed after induction of mixed tumors containing a 1:3 ratio of sst2-expressing cells to control cells. Tumor volume and incidence of metastases of mixed tumors were significantly reduced at day 13 post implantation. This effect decreased with time as at day 20, growth of mixed tumors was similar to that of control tumors. After administration of the cytotoxic somatostatin conjugate AN-238 on day 13, antitumor bystander effect observed in mixed tumors was significantly extended to day 20. We also observed that in vitro invasiveness of sst2-expressing PC-1.0 cells was significantly reduced. Tyrosine dephosphorylation of E-cadherin may participate in restoring the E-cadherin function, reducing in turn pancreatic cancer cell motility and invasiveness. This dephosphorylation depends on the tyrosine phosphatase src homology 2-containing tyrosine phosphatase 1 (SHP-1) positively coupled to sst2 receptor. The inhibitory effect of sst2 gene expression on pancreatic cancer growth and invasion combined with chemotherapy with targeted cytotoxic somatostatin analog administration provides a rationale for a therapeutic approach to gene therapy based on in vivo sst2 gene transfer.
Figures



References
-
- Lamberts S W, Krenning E P, Reubi J C. Endocr Rev. 1991;12:450–482. - PubMed
-
- Pollak M N, Schally A V. Proc Soc Exp Biol Med. 1998;217:143–152. - PubMed
-
- Arnold R, Frank M. Digestion. 1996;57, Suppl. 1:69–71. - PubMed
-
- Hoyer D, Bell G I, Berelowitz M, Epelbaum J, Feniuk W, Humphrey P P, AM, O C, Patel Y C, Schonbrunn A, Taylor J E, Reisine T. Trends Pharmacol Sci. 1995;16:86–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

