Redesign of substrate-selectivity determining modules of glutathione transferase A1-1 installs high catalytic efficiency with toxic alkenal products of lipid peroxidation
- PMID: 10900265
- PMCID: PMC16877
- DOI: 10.1073/pnas.150084897
Redesign of substrate-selectivity determining modules of glutathione transferase A1-1 installs high catalytic efficiency with toxic alkenal products of lipid peroxidation
Abstract
The evolution of proteins for novel functions involves point mutations and recombinations of domains or structural segments. Mimicking this process by rational design in vitro is still a major challenge. The present report demonstrates that the active site of the enzyme glutathione transferase (GST) A1-1 can be tailored for high catalytic efficiency with alkenals. The result is a >3,000-fold change in substrate selectivity involving a noteworthy change in preferred catalyzed reaction from aromatic nucleophilic substitution to Michael addition. The hydrophobic substrate binding pocket of GST A1-1 is formed by three structural modules, which were redesigned sequentially with four point mutations and the exchange of a helical segment. The substitutions were made to mimic first-sphere interactions with a substrate in GST A4-4, which naturally has high activity with alkenals. These substrates are toxic lipid peroxidation products of pathophysiological significance, and glutathione conjugation is a route of their inactivation. The final product of the sequential redesign of GST A1-1, mutant GIMFhelix, had a 300-fold increase in catalytic efficiency with nonenal and a >10 times decreased activity with 1-chloro-2,4-dinitrobenzene. In absolute values, GIMFhelix is more efficient than wild-type GST A4-4 with some alkenal substrates, with a k(cat)/K(m) value of 1.5 +/- 0. 1 10(6) M(-1) small middle dots(-1) for nonenal. The pKa value of the active-site Tyr-9 of GIMFhelix is 7.3 +/- 0.1, approaching the unusually low value of GST A4-4. Thus, rational redesign of the active-site region of an enzyme may be sufficient for the generation of efficient catalysts with altered chemical mechanism and novel selectivity.
Figures
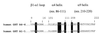

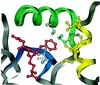

Comment in
-
Reengineering the glutathione S-transferase scaffold: a rational design strategy pays off.Proc Natl Acad Sci U S A. 2000 Sep 12;97(19):10298-300. doi: 10.1073/pnas.97.19.10298. Proc Natl Acad Sci U S A. 2000. PMID: 10984526 Free PMC article. No abstract available.
References
-
- Arnold F H, Volkov A A. Curr Opin Chem Biol. 1999;3:54–59. - PubMed
-
- Sinning I, Kleywegt G J, Cowan S W, Reinemer P, Dirr H W, Huber R, Gilliland G L, Armstrong R N, Ji X, Board P G, et al. J Mol Biol. 1993;232:192–212. - PubMed
-
- Mannervik B, Carlberg I, Larson K. In: Coenzymes and Cofactors. Dolphin D, Poulson R, Avramovic O, editors. 3B. New York: Wiley; 1989. pp. 475–516.
-
- Mannervik B, Jensson H. J Biol Chem. 1982;257:9909–9912. - PubMed
-
- Josephy P D, Mannervik B, Ortiz de Montellano P. Molecular Toxicology. New York: Oxford Univ. Press; 1997. pp. 152–186.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

