New roles for cis-jasmone as an insect semiochemical and in plant defense
- PMID: 10900270
- PMCID: PMC16867
- DOI: 10.1073/pnas.160241697
New roles for cis-jasmone as an insect semiochemical and in plant defense
Abstract
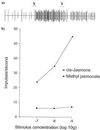
cis-jasmone, or (Z)-jasmone, is well known as a component of plant volatiles, and its release can be induced by damage, for example during insect herbivory. Using the olfactory system of the lettuce aphid to investigate volatiles from plants avoided by this insect, (Z)-jasmone was found to be electrophysiologically active and also to be repellent in laboratory choice tests. In field studies, repellency from traps was demonstrated for the damson-hop aphid, and with cereal aphids numbers were reduced in plots of winter wheat treated with (Z)-jasmone. In contrast, attractant activity was found in laboratory and wind tunnel tests for insects acting antagonistically to aphids, namely the seven-spot ladybird and an aphid parasitoid. When applied in the vapor phase to intact bean plants, (Z)-jasmone induced the production of volatile compounds, including the monoterpene (E)-beta-ocimene, which affect plant defense, for example by stimulating the activity of parasitic insects. These plants were more attractive to the aphid parasitoid in the wind tunnel when tested 48 h after exposure to (Z)-jasmone had ceased. This possible signaling role of (Z)-jasmone is qualitatively different from that of the biosynthetically related methyl jasmonate and gives a long-lasting effect after removal of the stimulus. Differential display was used to compare mRNA populations in bean leaves exposed to the vapor of (Z)-jasmone and methyl jasmonate. One differentially displayed fragment was cloned and shown by Northern blotting to be up-regulated in leaf tissue by (Z)-jasmone. This sequence was identified by homology as being derived from a gene encoding an alpha-tubulin isoform.
Figures



References
-
- Agrawal A A, Karban R. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 45–61.
-
- Shulaev V, Silverman P, Raskin I. Nature (London) 1997;385:718–721.
-
- Boland W, Hopke J, Piel J. In: Natural Product Analysis. Schreier P, Herderich M, Humpf H U, Schwab W, editors. Wiesbaden: Vieweg; 1998. pp. 255–269.
-
- Thaler J S, Stout M J, Karban R, Duffey S S. J Chem Ecol. 1996;22:1767–1781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

