Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency
- PMID: 10903343
- PMCID: PMC314308
- DOI: 10.1172/JCI9397
Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency
Abstract
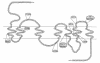
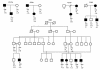
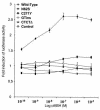
Over 20 severely obese subjects in 11 independent kindreds have been reported to have pathogenic heterozygous mutations in the gene encoding the melanocortin 4 receptor (MC4R), making this the most common known monogenic cause of human obesity. To date, the detailed clinical phenotype of this dominantly inherited disorder has not been defined, and no homozygous subjects have been described. We determined the nucleotide sequence of the entire coding region of the MC4R gene in 243 subjects with severe, early-onset obesity. A novel two-base pair GT insertion in codon 279 was found in two unrelated subjects, and four novel missense mutations, N62S, R165Q, V253I, C271Y, and one mutation (T112M) reported previously were found in five subjects. N62S was found in homozygous form in five children with severe obesity from a consanguineous pedigree. All four heterozygous carriers were nonobese. Several features of the phenotype, e.g. hyperphagia, tendency toward tall stature, hyperinsulinemia, and preserved reproductive function, closely resemble those reported previously in Mc4r knock-out mice. In addition, a marked increase in bone mineral density was seen in all affected subjects. In transient transfection assays, the N62S mutant receptor showed a responsiveness to alphaMSH that was intermediate between the wild-type receptor and mutant receptors carrying nonsense and missense mutations associated with dominantly inherited obesity. Thus MC4R mutations result in a syndrome of hyperphagic obesity in humans that can present with either dominant or recessive patterns of inheritance.
Figures
Comment in
-
Haploinsufficiency of the melanocortin-4 receptor: part of a thrifty genotype?J Clin Invest. 2000 Jul;106(2):185-7. doi: 10.1172/JCI10628. J Clin Invest. 2000. PMID: 10903333 Free PMC article. No abstract available.
References
-
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. - PubMed
-
- Krude H, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. - PubMed
-
- Ollmann MM, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. - PubMed
-
- Naggert JK, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. - PubMed
-
- Jackson RS, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

