Inhibition of mouse neuromuscular transmission and contractile function by okadaic acid and cantharidin
- PMID: 10903957
- PMCID: PMC1572180
- DOI: 10.1038/sj.bjp.0703418
Inhibition of mouse neuromuscular transmission and contractile function by okadaic acid and cantharidin
Abstract
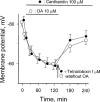
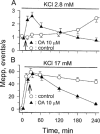
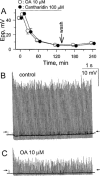
1. Phosphorylations of cellular proteins modulate biological activities. The effects of okadaic acid (0.1 - 10 microM) and cantharidin (1 - 100 microM), inhibitors of protein phosphatases, on the synaptic transmission at the mouse neuromuscular junction were explored. 2. Both inhibitors almost completely depressed twitch forces elicited by electrical stimulation of diaphragm muscles (the IC(50)s for okadaic acid and cantharidin were 1.1+/-0.2 and 13+/-1 microM, n=5, respectively) and suppressed contractures evoked by high K(+) and ryanodine more than 70%. Contractures caused by cardiotoxin, which destroys the integrity of sarcolemma, were not depressed. 3. Both okadaic acid (10 microM) and cantharidin (100 microM) depolarized muscle membranes from approximately -80 to approximately -60 mV in a partially reversible and tetrodotoxin-sensitive manner. The initial short-term enhancement of twitch responses (up to approximately 40%) was correlated with the inhibitors-induced repetitive firings of muscle action potential. 4. Treatment with either agent resulted in nearly complete inhibitions of endplate potential (epp). The IC(50)s were 0.8+/-0.2 and 9+/-2 microM (n=5), respectively, for okadaic acid and cantharidin. On high frequency stimulation, the coefficient of epps was increased more than 10 fold and the extent of epp run-down during stimulations intensified from approximately 25 to approximately 75%. Analyses of presynaptic quantal releases revealed decreases in epp quantal content and the immediately available vesicle pool. 5. The frequency of miniature epp was initially elevated up to 2 fold then suppressed down to approximately 30%. The small reduction in the amplitude was antagonized when the membrane of endplate area was repolarized. 6. The data suggest that okadaic acid and cantharidin inhibit mobilizations of synaptic vesicles and depress Ca(2+) release from sarcoplasmic reticulum and that protein phosphatases participate in the modulation of motor function.
Figures
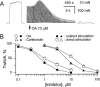


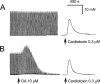
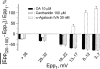
 at steady-state, the mean amplitude of the 20–100th epps (Epp(20–100)) during a train of stimulation, from the respective Epp1. The parameter signifies epp profiles (minus sign for run-down of train of epps, positive sign for epp run-up) and reflects overall facilitation (treated with ω-agatoxin IVA) or depression (with OA or cantharidin) of the mobilization of releasable transmitter quanta during intense stimulations. Data pooled from 5–6 preparations.
at steady-state, the mean amplitude of the 20–100th epps (Epp(20–100)) during a train of stimulation, from the respective Epp1. The parameter signifies epp profiles (minus sign for run-down of train of epps, positive sign for epp run-up) and reflects overall facilitation (treated with ω-agatoxin IVA) or depression (with OA or cantharidin) of the mobilization of releasable transmitter quanta during intense stimulations. Data pooled from 5–6 preparations.Similar articles
-
Evaluation of intrinsic modulation of synaptic transmission by ATP in mouse fast twitch muscle.J Neurophysiol. 1998 Nov;80(5):2550-8. doi: 10.1152/jn.1998.80.5.2550. J Neurophysiol. 1998. PMID: 9819262
-
Nicotinic actions of oxotremorine on murine skeletal muscle. Evidence against muscarinic modulation of acetylcholine release.Brain Res. 1990 Nov 26;534(1-2):142-8. doi: 10.1016/0006-8993(90)90124-t. Brain Res. 1990. PMID: 2073579
-
Reduction of quantal size and inhibition of neuromuscular transmission by bafilomycin A.Neuropharmacology. 2001 Oct;41(5):609-17. doi: 10.1016/s0028-3908(01)00104-6. Neuropharmacology. 2001. PMID: 11587716
-
Novel inhibition of contractility by wortmannin in skeletal muscle.Br J Pharmacol. 1998 Jul;124(5):849-56. doi: 10.1038/sj.bjp.0701898. Br J Pharmacol. 1998. PMID: 9692768 Free PMC article.
-
Studies on the contracture inducing action of triphenyltin in the mouse diaphragm.Eur J Pharmacol. 1994 Nov 1;292(1):95-101. doi: 10.1016/0926-6917(94)90031-0. Eur J Pharmacol. 1994. PMID: 7867695
Cited by
-
Identification of differentially expressed genes in SHSY5Y cells exposed to okadaic acid by suppression subtractive hybridization.BMC Genomics. 2012 Jan 27;13:46. doi: 10.1186/1471-2164-13-46. BMC Genomics. 2012. PMID: 22284234 Free PMC article.
-
Is protein phosphatase inhibition responsible for the toxic effects of okadaic Acid in animals?Toxins (Basel). 2013 Feb 4;5(2):267-85. doi: 10.3390/toxins5020267. Toxins (Basel). 2013. PMID: 23381142 Free PMC article. Review.
References
-
- AHERN G.P., JUNANKAR P.R., DULHUNTY A.F. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994;352:369–374. - PubMed
-
- ALBUQUERQUE E.X., DESHPANDE S.S., ARACAVA Y., ALKONDON M., DALY J.W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. FEBS Lett. 1986;199:113–120. - PubMed
-
- ALBUQUERQUE E.X., SCHUH F.T., KAUFFMAN F.C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflügers Arch. 1971;328:36–50. - PubMed
-
- ÄMMÄLÄ C., ELIASSON L., BOKVIST K., BERGGREN P.-O., HONKANEN R.E., SJÖHOLM Å., RORSMAN P. Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic β-cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4343–4347. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

