Sodium reabsorption in thick ascending limb of Henle's loop: effect of potassium channel blockade in vivo
- PMID: 10903963
- PMCID: PMC1572189
- DOI: 10.1038/sj.bjp.0703429
Sodium reabsorption in thick ascending limb of Henle's loop: effect of potassium channel blockade in vivo
Abstract
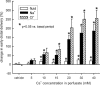
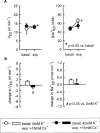
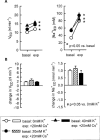
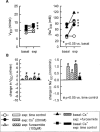
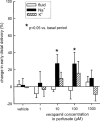
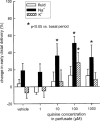
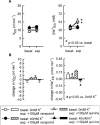
1. Based on previous in vitro studies, inhibition of K(+) recycling in thick ascending limb (TAL) is expected to lower Na(+) reabsorption through (i) reducing the luminal availability of K(+) to reload the Na(+)-2Cl(-)-K(+) cotransporter and (ii) diminishing the lumen positive transepithelial potential difference which drives paracellular cation transport. 2. This issue was investigated in anaesthetized rats employing microperfusion of Henle's loop downstream from late proximal tubular site with K(+)-free artificial tubular fluid in nephrons with superficial glomeruli. 3. The unselective K(+) channel blocker Cs(+) (5 - 40 mM) dose-dependently increased early distal tubular delivery of fluid and Na(+) with a maximum increase of approximately 20 and 185%, respectively, indicating predominant effects on water-impermeable TAL. 4. The modest inhibition of Na(+) reabsorption in response to the 15 mM of Cs(+) but not the enhanced inhibition by 20 mM Cs(+) was prevented by luminal K(+) supplementation. Furthermore, pretreatment with 20 mM Cs(+) did not attenuate the inhibitory effect of furosemide (100 microM) on Na(+)-2Cl(-)-K(+) cotransport. 5. Neither inhibitors of large (charybdotoxin 1 microM) nor low (glibenclamide 250 microM; U37883A 100 microM) conductance K(+) channels altered loop of Henle fluid or Na(+) reabsorption. 6. The intermediate conductance K(+) channel blockers verapamil and quinine (100 microM) modestly increased early distal tubular Na(+) but not fluid delivery, indicating a role for this K(+) channel in Na(+) reabsorption in TAL. As observed for equieffective concentrations of Cs(+) (15 mM), Na(+) reabsorption was preserved by K(+) supplementation. 7. The results indicate that modest inhibition of K(+) channels lowers the luminal availability of K(+) and thus transcellular Na(+) reabsorption in TAL. More complete inhibition lowers paracellular Na(+) transport probably by reducing or even abolishing the lumen positive transepithelial potential difference. Under the latter conditions, transcellular Na(+) transport may be restored by paracellular K(+) backleak.
Figures
Similar articles
-
Eukaliuric diuresis and natriuresis in response to the KATP channel blocker U37883A: micropuncture studies on the tubular site of action.Br J Pharmacol. 1999 Aug;127(8):1811-8. doi: 10.1038/sj.bjp.0702742. Br J Pharmacol. 1999. PMID: 10482911 Free PMC article.
-
Intratubular application of sodium azide inhibits loop of Henle reabsorption and tubuloglomerular feedback response in anesthetized rats.Naunyn Schmiedebergs Arch Pharmacol. 1998 Sep;358(3):367-73. doi: 10.1007/pl00005266. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9774225
-
Evidence supporting a role for KCl cotransporter in the thick ascending limb of Henle's loop.Kidney Int. 2001 Nov;60(5):1809-23. doi: 10.1046/j.1523-1755.2001.00994.x. Kidney Int. 2001. PMID: 11703599
-
Bicarbonate transport along the loop of Henle: molecular mechanisms and regulation.J Nephrol. 2002 Mar-Apr;15 Suppl 5:S88-96. J Nephrol. 2002. PMID: 12027225 Review.
-
Role of Ca2+-activated K+ channel in regulation of NaCl reabsorption in thick ascending limb of Henle's loop.Comp Biochem Physiol A Comp Physiol. 1988;90(4):757-65. doi: 10.1016/0300-9629(88)90695-0. Comp Biochem Physiol A Comp Physiol. 1988. PMID: 2902984 Review.
Cited by
-
Insulin-induced electrophysiology changes in human pleura are mediated via its receptor.Exp Diabetes Res. 2010;2010:853176. doi: 10.1155/2010/853176. Epub 2010 Aug 12. Exp Diabetes Res. 2010. PMID: 20814548 Free PMC article.
-
Micropuncturing the nephron.Pflugers Arch. 2009 May;458(1):189-201. doi: 10.1007/s00424-008-0581-7. Epub 2008 Aug 28. Pflugers Arch. 2009. PMID: 18752000 Free PMC article. Review.
-
Recording ion channels in isolated, split-opened tubules.Methods Mol Biol. 2013;998:341-53. doi: 10.1007/978-1-62703-351-0_27. Methods Mol Biol. 2013. PMID: 23529443 Free PMC article.
References
-
- ARRUDA J.A.L. Characterization of the effect of quinine on Na+ transport by the toad and turtle bladders. J. Pharmacol. Exp. Ther. 1983;224:297–301. - PubMed
-
- BAILLY C. Transducing pathways involved in the control of NaCl reabsorption in the thick ascending limb of Henle's loop. Kidney. Int. 1998;65 Suppl:S29–S35. - PubMed
-
- BLEICH M., SCHLATTER E., GREGER R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pflügers. Arch. 1990;415:449–460. - PubMed
-
- GREGER R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol. Rev. 1985;65:760–797. - PubMed
-
- GREGER R., SCHLATTER E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers. Arch. 1981;392:92–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

