Partial characterization of a 17 kDa protein of Clonorchis sinensis
- PMID: 10905071
- PMCID: PMC2721119
- DOI: 10.3347/kjp.2000.38.2.95
Partial characterization of a 17 kDa protein of Clonorchis sinensis
Abstract
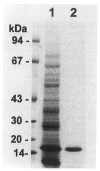
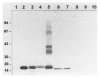
A 17 kDa protein from Clonorchis sinensis adults was purified by a procedure including Sephacryl S-200 HR gel filtration and Q-Sepharose anion exchange chromatography. The protein was proved to be a cysteine protease as it showed hydrolytic activity toward Cbz-Phe-Arg-AMC in the presence of dithiothreitol and was inhibited by specific inhibitors such as iodoacetic acid or trans epoxy-succinly-L-leucyl-amido(4-guanidino) butane. The polyclonal antibody raised against the protein reacted to 17 kDa proteins of trematodes such as Paragonimus westermani, Fasciola hepatica, Opisthorchis viverrini, Gymnophalloides seoi, and Metagonimus yokogawai. The antibody recognized the 17 kDa and 16 kDa cysteine proteases purified from C. sinensis, P. westermani, and G. seoi as well. These results suggest that the 17 kDa protein may be a cysteine protease commonly present in trematodes.
Figures
References
-
- McKerrow JH. Minireview: Parasite proteases. Exp Parasitol. 1989;68:111–115. - PubMed
-
- Choi MH, Chai JY, Lee SH. Purification and characterization of a 16-kDa cysteine proteinase of Gymnophalloides seoi (Gymnophallidae)metacercariae. J Parasitol. 1998;84:350–355. - PubMed
-
- Chung YB, Kong Y, Yang HJ, Kang SY, Cho SY. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol. 1997;83:902–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

