Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction
- PMID: 10906173
- PMCID: PMC112240
- DOI: 10.1128/jvi.74.16.7196-7203.2000
Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction
Abstract
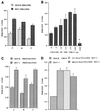
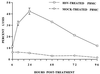
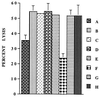
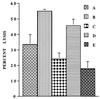
Infections with herpes simplex virus type 1 (HSV-1) in humans and in animal models are accompanied by enhanced natural killer (NK) activity. In vitro, HSV-1 also enhances the NK activity of human peripheral blood mononuclear cells (PBMC). The molecular basis of this enhanced NK activity, however, is not well characterized. We investigated the role of human interleukin-15 (IL-15) in this phenomenon and report here that HSV-1-mediated enhanced NK activity was abrogated by neutralizing antibodies for IL-15 but not for other cytokines (i.e., IL-2, IL-12, gamma interferon [IFN-gamma], tumor necrosis factor alpha, or IFN-alpha). Anti-CD122 antibodies which block signaling through IL-2 receptor beta chain, and therefore neutralize the effects of IL-15 (and IL-2), also abrogated this enhancement. Furthermore, HSV-1 increased the levels of IL-15 mRNA and the production of IL-15 in HSV-1-infected PBMC cultures. The neutralization of IL-15 in cocultures of PBMC with HSV-1-infected cells significantly increased HSV-1 production. These results strongly suggest a role for IL-15 in the HSV-1-mediated in vitro enhancement of NK activity and in the PBMC-mediated suppression of HSV-1 replication.
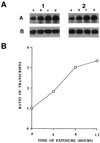
Figures

References
-
- Ahmad A, Menezes J. The binding of Epstein-Barr virus to human platelets causes the release of transforming growth factor-β. J Immunol. 1997;159:3984–3988. - PubMed
-
- Ahmad A, Menezes J. Defective killing activity against gp120/41-expressing human erythroleukaemic K562 cell line by monocytes and natural killer cells from HIV-infected individuals. AIDS. 1996;10:143–149. - PubMed
-
- Atedzoé B N, Ahmad A, Menezes J. Enhancement of natural killer cell cytotoxicity by the human herpesvirus-7 via IL-15 induction. J Immunol. 1997;159:4966–4972. - PubMed
-
- Bamford R N, Grant A J, Burton J D, Peters C, Kuzys G, Goldman C D, Brennan J, Roessler E, Waldman T A. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T cell proliferation and the induction of lymphokine activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. - PMC - PubMed
-
- Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

