Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region
- PMID: 10906187
- PMCID: PMC112254
- DOI: 10.1128/jvi.74.16.7338-7348.2000
Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region
Erratum in
- J Virol 2002 Jul;76(13):6864
Abstract
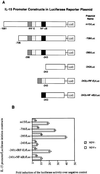
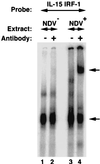
We identified an interferon regulatory factor motif (IRF-E) upstream of an NF-kappaB binding site in the interleukin-15 (IL-15) promoter. Since these two motifs are part of the virus-inducible enhancer region of the beta interferon promoter, we speculated that there might be similar responses of these two genes to stimuli such as viruses. To test this hypothesis, L929 cells were infected with Newcastle disease virus (NDV), which led to the induction of IL-15 mRNA and protein expression. Using IL-15 promoter-reporter deletion constructs, a virus-inducible region, encompassing IRF-E, NF-kappaB, and a 13-nucleotide sequence flanked by these two motifs, was mapped to the -295-to--243 position relative to the transcription initiation site. Using cotransfection studies, it was demonstrated that all three motifs were essential to achieve the maximum promoter activity induced by IRF-1 and NF-kappaB expression plasmids. The presence of a virus-inducible region in the IL-15 promoter suggests a role for IL-15 as a component of host antiviral defense mechanisms.
Figures


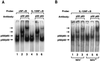





References
-
- Armitage R J, Macduff B M, Eisenman J, Paxton R, Grabstein K H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. - PubMed
-
- Au W-C, Moore P A, Lafleur D W, Tombal B, Pitha P M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273:29210–29217. - PubMed
-
- Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

