Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail
- PMID: 10906204
- PMCID: PMC112271
- DOI: 10.1128/jvi.74.16.7508-7517.2000
Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail
Abstract
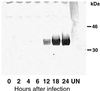
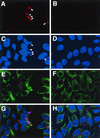
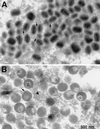
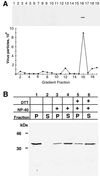
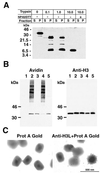
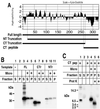
The vaccinia virus H3L open reading frame encodes a 324-amino-acid immunodominant membrane component of virus particles. Biochemical and microscopic studies demonstrated that the H3L protein was expressed late in infection, accumulated in the cytoplasmic viral factory regions, and associated primarily with amorphous material near immature virions and with intracellular virion membranes. Localization of the H3L protein on the surfaces of viral particles and anchorage via the hydrophobic tail were consistent with its extraction by NP-40 in the absence of reducing agents, its trypsin sensitivity, its reactivity with a membrane-impermeable biotinylation reagent, and its immunogold labeling with an antibody to a peptide comprising amino acids 247 to 259. The H3L protein, synthesized in a coupled in vitro transcription/translation system, was tightly anchored to membranes as determined by resistance to Na(2)CO(3) (pH 11) extraction and cytoplasmically oriented as shown by sensitivity to proteinase K digestion. Further studies demonstrated that membrane insertion of the H3L protein occurred posttranslationally and that the C-terminal hydrophobic domain was necessary and sufficient for this to occur. These data indicated that the H3L protein is a member of the C-terminal anchor family and supported a model in which it is synthesized on free ribosomes and inserts into the membranes of viral particles during their maturation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

