Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication
- PMID: 10906205
- PMCID: PMC112272
- DOI: 10.1128/jvi.74.16.7518-7528.2000
Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication
Abstract
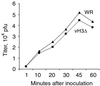
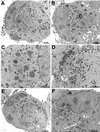
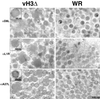
The C-terminal membrane anchor protein encoded by the H3L open reading frame of vaccinia virus is located on the surfaces of intracellular mature virions. To investigate the role of the H3L protein, we constructed deletion (vH3Delta) and inducible (vH3i) null mutants. The H3L protein was not detected in lysates of cells infected with vH3Delta or vH3i in the absence of inducer. Under these conditions, plaques were small and round instead of large and comet shaped, indicative of decreased virus replication or cell-to-cell spread. The mutant phenotype was correlated with reduced yields of infectious intra- and extracellular virus in one-step growth experiments. The defect in vH3i replication could not be attributed to a role of the H3L protein in virus binding, internalization, or any event prior to late gene expression. Electron microscopic examination of cells infected with vH3Delta or vH3i in the absence of inducer revealed that virion assembly was impaired, resulting in a high ratio of immature to mature virus forms with an accumulation of crescent membranes adjacent to granular material and DNA crystalloids. The absence of the H3L protein did not impair the membrane localization of virion surface proteins encoded by the A27L, D8L, and L1R genes. The wrapping of virions and actin tail formation were not specifically blocked, but there was an apparent defect in low-pH-mediated syncytium formation that could be attributed to decreased virus particle production. The phenotypes of the H3L deletion and repression mutants were identical to each other but differed from those produced by null mutations of genes encoding other vaccinia virus membrane components.
Figures




References
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Boulter E A, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

