The structure of cucumber mosaic virus and comparison to cowpea chlorotic mottle virus
- PMID: 10906212
- PMCID: PMC112279
- DOI: 10.1128/jvi.74.16.7578-7586.2000
The structure of cucumber mosaic virus and comparison to cowpea chlorotic mottle virus
Abstract
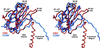
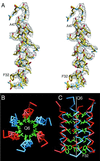
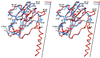
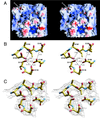
The structure of cucumber mosaic virus (CMV; strain Fny) has been determined to a 3.2-A resolution using X-ray crystallography. Despite the fact that CMV has only 19% capsid protein sequence identity (34% similarity) to cowpea chlorotic mottle virus (CCMV), the core structures of these two members of the Bromoviridae family are highly homologous. As suggested by a previous low-resolution structural study, the 305-A diameter (maximum) of CMV is approximately 12 A larger than that of CCMV. In CCMV, the structures of the A, B, and C subunits are nearly identical except in their N termini. In contrast, the structures of two loops in subunit A of CMV differ from those in B and C. These loops are 6 and 7 residues longer than the analogous regions in CCMV. Unlike that of CCMV, the capsid of CMV does not undergo swelling at pH 7.0 and is stable at pH 9.0. This may be partly due to the fact that the N termini of the B and C subunits form a unique bundle of six amphipathic helices oriented down into the virion core at the threefold axes. In addition, while CCMV has a cluster of aspartic acid residues at the quasi-threefold axis that are proposed to bind metal in a pH-dependent manner, this cluster is replaced by complementing acids and bases in CMV. Finally, this structure clearly demonstrates that the residues important for aphid transmission lie at the outermost portion of the betaH-betaI loop and yields details of the portions of the virus that are hypothesized to mediate binding to aphid mouthparts.
Figures



References
-
- Abad-Zapatero C, Abdel-Meguid S S, Johnson J E, Leslie A G W, Rayment I, Rossmann M G, Suck D, Tsukihara T. Structure of southern bean mosaic virus at 2.8Å resolution. Nature (London) 1980;286:33–39. - PubMed
-
- Bailey S. The CCP4 suite: programs for protein crystallography. Acta Crystallogr Sect D. 1994;50:760–763. - PubMed
-
- Bancroft J B, Hills G J, Markham R. A study of the self-assembly process in a small spherical virus. Formation of organized structures from protein subunits in vitro. Virology. 1967;31:354–379. - PubMed
-
- Collaborative Computational Project n. The CCP4 suite: programs for protein crystallography. Acta Crystallogr Sect D. 1994;50:760–763. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

