Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections
- PMID: 10906215
- PMCID: PMC112282
- DOI: 10.1128/jvi.74.16.7610-7618.2000
Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections
Abstract
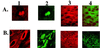
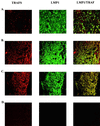
Human herpesviruses are characterized by distinct states of infection. Typically in permissive herpesvirus infection, abundant virus production results in cell lysis. In latent transforming Epstein-Barr virus (EBV) infection, viral proteins that induce cell growth are expressed. The immunodeficiency-associated hairy leukoplakia (HLP) lesion is the only pathologic manifestation of permissive EBV infection; however, within HLP, viral proteins characteristic of latent infection have also been detected. In this study, we further analyzed expression of EBV latent genes and investigated their contribution to the unique histologic phenotype of HLP. Coexpression of lytic and transforming viral proteins was detected simultaneously within individual HLP keratinocytes. LMP1 has now been shown to be uniformly expressed in the affected tissue, and it is associated and colocalizes with tumor necrosis factor receptor-associated factor (TRAF) signaling molecules. Effects induced by activated TRAF signaling that were detected in HLP included activation of NF-kappaB and c-Jun terminal kinase 1 (JNK1) and upregulated expression of epidermal growth factor receptor (EGFR), CD40, A20, and TRAFs. This study identifies a novel state of EBV infection with concurrent expression of replicative and transforming proteins. It is probable that both replicative and latent proteins contribute to HLP development and induce many of the histologic features of HLP, such as acanthosis and hyperproliferation. In contrast to other permissive herpesvirus infections, expression of EBV transforming proteins within the permissively infected HLP tissue enables epithelial cell survival and may enhance viral replication.
Figures





Similar articles
-
Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia.Virology. 1998 Aug 15;248(1):53-65. doi: 10.1006/viro.1998.9268. Virology. 1998. PMID: 9705255
-
Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions.Oral Dis. 2002;8 Suppl 2:161-8. doi: 10.1034/j.1601-0825.2002.00028.x. Oral Dis. 2002. PMID: 12164651 Review.
-
Epstein-Barr virus coinfection and recombination in non-human immunodeficiency virus-associated oral hairy leukoplakia.J Infect Dis. 1995 May;171(5):1122-30. doi: 10.1093/infdis/171.5.1122. J Infect Dis. 1995. PMID: 7751686
-
Persistent productive Epstein-Barr virus replication in normal epithelial cells in vivo.J Infect Dis. 2001 Dec 15;184(12):1499-507. doi: 10.1086/323992. Epub 2001 Dec 3. J Infect Dis. 2001. PMID: 11740724
-
Current Trends and Alternative Scenarios in EBV Research.Methods Mol Biol. 2017;1532:1-32. doi: 10.1007/978-1-4939-6655-4_1. Methods Mol Biol. 2017. PMID: 27873264 Review.
Cited by
-
Epstein-Barr Virus and Its Association with Oral Hairy Leukoplakia: A Short Review.Int J Dent. 2016;2016:4941783. doi: 10.1155/2016/4941783. Epub 2016 Mar 7. Int J Dent. 2016. PMID: 27047546 Free PMC article. Review.
-
Induction of Epstein-Barr virus latent membrane protein 1 by a lytic transactivator Rta.J Virol. 2004 Dec;78(23):13028-36. doi: 10.1128/JVI.78.23.13028-13036.2004. J Virol. 2004. PMID: 15542654 Free PMC article.
-
IRF7 activation by Epstein-Barr virus latent membrane protein 1 requires localization at activation sites and TRAF6, but not TRAF2 or TRAF3.Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18448-53. doi: 10.1073/pnas.0809933105. Epub 2008 Nov 18. Proc Natl Acad Sci U S A. 2008. PMID: 19017798 Free PMC article.
-
Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells.PLoS Pathog. 2015 Oct 2;11(10):e1005195. doi: 10.1371/journal.ppat.1005195. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26431332 Free PMC article.
-
The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1.PLoS Pathog. 2012 Feb;8(2):e1002516. doi: 10.1371/journal.ppat.1002516. Epub 2012 Feb 9. PLoS Pathog. 2012. PMID: 22346751 Free PMC article.
References
-
- Busson P, Zhang Q, Guillon J M, Gregory C D, Young L S, Clausse B, Lipinski M, Rickinson A B, Tursz T. Elevated expression of ICAM1 (CD54) and minimal expression of LFA3 (CD58) in Epstein-Barr-virus-positive nasopharyngeal carcinoma cells. Int J Cancer. 1992;50:863–867. - PubMed
-
- Chrysomali E, Greenspan J S, Dekker N, Greenspan D, Regezi J A. Apoptosis-associated proteins in oral hairy leukoplakia. Oral Dis. 1996;2:279–284. - PubMed
-
- Dawson C W, Eliopoulos A G, Dawson J, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, disturbs epithelial cell differentiation. Oncogene. 1995;10:69–77. - PubMed
-
- Dawson C W, Rickinson A B, Young L S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990;344:777–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

