Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes
- PMID: 10906225
- PMCID: PMC112292
- DOI: 10.1128/jvi.74.16.7678-7682.2000
Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes
Abstract
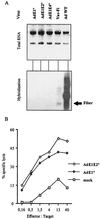
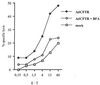
We previously demonstrated that a single injection of 10(9) PFU of recombinant adenovirus into patients induces strong vector-specific immune responses (H. Gahéry-Ségard, V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J.-G. Guillet, and F. Farace, J. Clin. Investig. 100:2218-2226, 1997). In the present study we analyzed the mechanism of vector recognition by cytotoxic T lymphocytes (CTL). CD8(+) CTL lines were derived from two patients and maintained in long-term cultures. Target cell infections with E1-deleted and E1-plus E2-deleted adenoviruses, as well as transcription-blocking experiments with actinomycin D, revealed that host T-cell recognition did not require viral gene transcription. Target cells treated with brefeldin A were not lysed, indicating that viral input protein-derived peptides are associated with HLA class I molecules. Using recombinant capsid component-loaded targets, we observed that the three major proteins could be recognized. These results raise the question of the use of multideleted adenoviruses for gene therapy in the quest to diminish antivector CTL responses.
Figures


References
-
- Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. - PubMed
-
- Crystal R, McElvaney N, Rosenfeld M, Chu C, Mastrangeli A, Hay J, Brody S, Jaffe H, Eissa N, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. - PubMed
-
- Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

