Molecular characterization at the RNA and gene levels of U3 snoRNA from a unicellular green alga, Chlamydomonas reinhardtii
- PMID: 10908360
- PMCID: PMC102673
- DOI: 10.1093/nar/28.15.2959
Molecular characterization at the RNA and gene levels of U3 snoRNA from a unicellular green alga, Chlamydomonas reinhardtii
Abstract
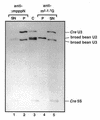
A U3 snoRNA gene isolated from a Chlamydomonas reinhardtii (CRE:) genomic library contains putative pol III-specific transcription signals similar to those of RNA polymerase III-specific small nuclear (sn)RNA genes of higher plants. The 222 nt long CRE: U3 snoRNA was immunoprecipitated by anti-gamma-mpppN antisera, but not by anti-m(2,2,7)G antibodies, supporting the notion that it is a RNA polymerase III transcript. Tagged CRE: U3 snoRNA gene constructs were expressed in CRE: cells. Results of chemical and enzymatic structure probing of CRE: U3 snoRNA in solution and of DMS modification of CRE: U3 snoRNA under in vivo conditions revealed that the two-hairpin structure of the 5'-domain that is found in solution is no longer detected under in vivo conditions. The observed differences can be explained by the formation of several base pair interactions with the 18S and 5'-ETS parts of the pre-rRNA. A model that involves five intermolecular helices is proposed.
Figures


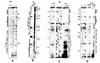
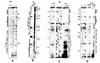


References
-
- Eichler D.C. and Craig,N. (1994) Prog. Nucleic Acid Res. Mol. Biol., 49, 197–239. - PubMed
-
- Maxwell E.S. and Fournier,M.J. (1995) Annu. Rev. Biochem., 64, 897–934. - PubMed
-
- Bachellerie J.P. and Cavaillé,J. (1998) In Grosjean,H. and Benne,R. (eds), The Modification and Editing of RNA. ASM Press, Washington, DC, pp. 255–272.
-
- Ofengand J. and Fournier,M.J. (1998) In Grosjean,H. and Benne,R. (eds), The Modification and Editing of RNA. ASM Press, Washington, DC, pp. 229–253.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

