HIV-1 rev depolymerizes microtubules to form stable bilayered rings
- PMID: 10908577
- PMCID: PMC2180222
- DOI: 10.1083/jcb.150.2.349
HIV-1 rev depolymerizes microtubules to form stable bilayered rings
Abstract
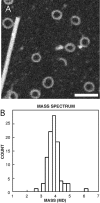
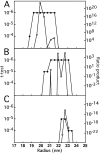
We describe a novel interaction between HIV-1 Rev and microtubules (MTs) that results in the formation of bilayered rings that are 44-49 nm in external diameter, 3.4-4.2 MD (megadaltons) in mass, and have 28-, 30-, or 32-fold symmetry. Ring formation is not sensitive to taxol, colchicine, or microtubule-associated proteins, but requires Mg(2+) and is inhibited by maytansine. The interaction involves the NH(2)-terminal domain of Rev and the face of tubulin exposed on the exterior of the MTs. The NH(2)-terminal half of Rev has unexpected sequence similarity to the tubulin-binding portion of the catalytic/motor domains of the microtubule-destabilizing Kin I kinesins. We propose a model wherein binding of Rev dimers to MTs at their ends causes segments of two neighboring protofilaments to peel off and close into rings, circumferentially containing 14, 15, or 16 tubulin heterodimers, with Rev bound on the inside. Rev has a strong inhibitory effect on aster formation in Xenopus egg extracts, demonstrating that it can interact with tubulin in the presence of normal levels of cellular constituents. These results suggest that Rev may interact with MTs to induce their destabilization, a proposition consistent with the previously described disruption of MTs after HIV-1 infection.
Figures




References
-
- Auer M., Gremlich H.-U., Seifert J.-M., Daly T.J., Parslow T.G., Casari G., Gstach H. Helix-loop-helix motif in HIV-1 Rev. Biochemistry. 1994;33:2988–2996. - PubMed
-
- Bai R., Taylor G.F., Schmidt J.M., Williams M.D., Kepler J.A., Pettit G.R., Hamel E. Interaction of dolastatin 10 with tubulininduction of aggregation and binding and dissociation reactions. Mol. Pharmacol. 1995;47:965–976. - PubMed
-
- Bai R., Schwartz R.E., Kepler J.A., Pettit G.R., Hamel E. Characterization of the interaction of cryptophycin 1 with tubulinbinding in the Vinca domain, competitive inhibition of dolastatin 10 binding, and an unusual aggregation reaction. Cancer Res. 1996;56:4398–4406. - PubMed
-
- Bai R., Durso N.A., Sackett D.L., Hamel E. Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulincomparison with dolastatin 10 and cryptophycin 1. Biochemistry. 1999;38:14302–14310. - PubMed
-
- Battiste J.L., Mao H.Y., Rao N.S., Tan R.Y., Muhandiram D.R., Kay L.E., Frankel A.D., Williamson J.R. Alpha helix-RNA major groove recognition in an HIV-1 Rev peptide RRE RNA complex. Science. 1996;273:1547–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

