Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse
- PMID: 10908596
- PMCID: PMC6772523
- DOI: 10.1523/JNEUROSCI.20-15-05594.2000
Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse
Abstract
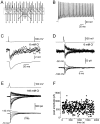
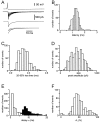
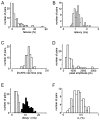
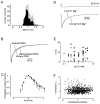
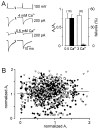
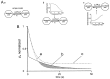
We have examined factors that determine the strength and dynamics of GABAergic synapses between interneurons [dentate gyrus basket cells (BCs)] and principal neurons [dentate gyrus granule cells (GCs)] using paired recordings in rat hippocampal slices at 34 degrees C. Unitary IPSCs recorded from BC-GC pairs in high intracellular Cl(-) concentration showed a fast rise and a biexponential decay, with mean time constants of 2 and 9 msec. The mean quantal conductance change, determined directly at reduced extracellular Ca(2+)/Mg(2+) concentration ratios, was 1.7 nS. Quantal release at the BC-GC synapse occurred with short delay and was highly synchronized. Analysis of IPSC peak amplitudes and numbers of failures by multiple probability compound binomial analysis indicated that synaptic transmission at the BC-GC synapse involves three to seven release sites, each of which releases transmitter with high probability ( approximately 0.5 in 2 mm Ca(2+)/1 mm Mg(2+)). Unitary BC-GC IPSCs showed paired-pulse depression (PPD); maximal depression, measured for 10 msec intervals, was 37%, and recovery from depression occurred with a time constant of 2 sec. Paired-pulse depression was mainly presynaptic in origin but appeared to be independent of previous release. Synaptic transmission at the BC-GC synapse showed frequency-dependent depression, with half-maximal decrease at 5 Hz after a series of 1000 presynaptic action potentials. The relative stability of transmission at the BC-GC synapse is consistent with a model in which an activity-dependent gating mechanism reduces release probability and thereby prevents depletion of the releasable pool of synaptic vesicles. Thus several mechanisms converge on the generation of powerful and sustained transmission at interneuron-principal neuron synapses in hippocampal circuits.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
