Clinical mutations in the L1 neural cell adhesion molecule affect cell-surface expression
- PMID: 10908608
- PMCID: PMC6772530
- DOI: 10.1523/JNEUROSCI.20-15-05696.2000
Clinical mutations in the L1 neural cell adhesion molecule affect cell-surface expression
Abstract
Mutations in the L1 neural cell adhesion molecule, a transmembrane glycoprotein, cause a spectrum of congenital neurological syndromes, ranging from hydrocephalus to mental retardation. Many of these mutations are single amino acid changes that are distributed throughout the various domains of the protein. Defective herpes simplex virus vectors were used to express L1 protein with the clinical missense mutations R184Q and D598N in the Ig2 and Ig6 extracellular domains, respectively, and S1194L in the cytoplasmic domain. All three mutant proteins were expressed at similar levels in infected cells. Neurite outgrowth of cerebellar granule cells was stimulated on astrocytes expressing wild-type or S1194L L1, whereas those expressing R184Q and D598N L1 failed to increase neurite length. Live cell immunofluorescent staining of L1 demonstrated that most defective vector-infected cells did not express R184Q or D598N L1 on their cell surface. This greatly diminished cell-surface expression occurred in astrocytes, neurons, and non-neural cells. In contrast to wild-type or S1194L L1, the R184Q and D598N L1 proteins had altered apparent molecular weights and remained completely endoglycosidase H (endoH)-sensitive, suggesting incomplete post-translational processing. We propose that some missense mutations in human L1 impede correct protein trafficking, with functional consequences independent of protein activity. This provides a rationale for how expressed, full-length proteins with single amino acid changes could cause clinical phenotypes similar in severity to knock-out mutants.
Figures
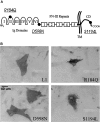

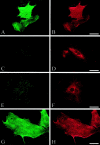



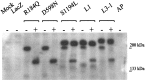
References
-
- Aridor M, Bannykh SI, Rowe T, Balch WE. Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J Biol Chem. 1999;274:4389–4399. - PubMed
-
- Asou H, Miura M, Kobayashi M, Uyemura K. The cell adhesion molecule L1 has a specific role in neural cell migration. NeuroReport. 1992;3:481–484. - PubMed
-
- Brummendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
