Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease
- PMID: 10908610
- PMCID: PMC6772529
- DOI: 10.1523/JNEUROSCI.20-15-05709.2000
Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease
Abstract
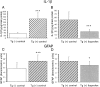
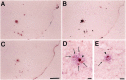
The brain in Alzheimer's disease (AD) shows a chronic inflammatory response characterized by activated glial cells and increased expression of cytokines and complement factors surrounding amyloid deposits. Several epidemiological studies have demonstrated a reduced risk for AD in patients using nonsteroidal anti-inflammatory drugs (NSAIDs), prompting further inquiries about how NSAIDs might influence the development of AD pathology and inflammation in the CNS. We tested the impact of chronic orally administered ibuprofen, the most commonly used NSAID, in a transgenic model of AD displaying widespread microglial activation, age-related amyloid deposits, and dystrophic neurites. These mice were created by overexpressing a variant of the amyloid precursor protein found in familial AD. Transgene-positive (Tg+) and negative (Tg-) mice began receiving chow containing 375 ppm ibuprofen at 10 months of age, when amyloid plaques first appear, and were fed continuously for 6 months. This treatment produced significant reductions in final interleukin-1beta and glial fibrillary acidic protein levels, as well as a significant diminution in the ultimate number and total area of beta-amyloid deposits. Reductions in amyloid deposition were supported by ELISA measurements showing significantly decreased SDS-insoluble Abeta. Ibuprofen also decreased the numbers of ubiquitin-labeled dystrophic neurites and the percentage area per plaque of anti-phosphotyrosine-labeled microglia. Thus, the anti-inflammatory drug ibuprofen, which has been associated with reduced AD risk in human epidemiological studies, can significantly delay some forms of AD pathology, including amyloid deposition, when administered early in the disease course of a transgenic mouse model of AD.
Figures


References
-
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. - PubMed
-
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APPSw transgenic mice. Neurobiol Aging. 2000;20:581–589. - PubMed
-
- Breitner JCS, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, Pericak-Vance MA, Saunders AM. Delayed onset of Alzheimer's disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging. 1995;16:523–530. - PubMed
-
- Dickson DW, Lee SC, Mattiace LA, Yen S-HC, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993;7:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
