Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo
- PMID: 10908615
- PMCID: PMC6772559
- DOI: 10.1523/JNEUROSCI.20-15-05748.2000
Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo
Abstract
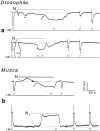
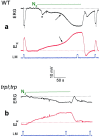
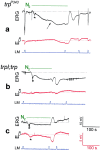
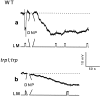
Drosophila transient receptor potential (TRP) is a prototypical member of a novel family of channel proteins underlying phosphoinositide-mediated Ca(2+) entry. Although the initial stages of this signaling cascade are well known, downstream events leading to the opening of the TRP channels are still obscure. In the present study we applied patch-clamp whole-cell recordings and measurements of Ca(2+) concentration by ion-selective microelectrodes in eyes of normal and mutant Drosophila to isolate the TRP and TRP-like (TRPL)-dependent currents. We report that anoxia rapidly and reversibly depolarizes the photoreceptors and induces Ca(2+) influx into these cells in the dark. We further show that openings of the light-sensitive channels, which mediate these effects, can be obtained by mitochondrial uncouplers or by depletion of ATP in photoreceptor cells, whereas the effects of illumination and all forms of metabolic stress were additive. Effects similar to those found in wild-type flies were also found in mutants with strong defects in rhodopsin, Gq-protein, or phospholipase C, thus indicating that the metabolic stress operates at a late stage of the phototransduction cascade. Genetic elimination of both TRP and TRPL channels prevented the effects of anoxia, mitochondrial uncouplers, and depletion of ATP, thus demonstrating that the TRP and TRPL channels are specific targets of metabolic stress. These results shed new light on the properties of the TRP and TRPL channels by showing that a constitutive ATP-dependent process is required to keep these channels closed in the dark, a requirement that would make them sensitive to metabolic stress.
Figures



References
-
- Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res. 1999;42:543–549. - PubMed
-
- Baumann F, Mauro A. Effect of hypoxia on the change in membrane conductance evoked by illumination in arthropod photoreceptors. Nat New Biol. 1973;244:146–148. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. Signal transduction: the calcium entry pas de deux. Science. 2000;287:1604–1605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
