BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway
- PMID: 10908618
- PMCID: PMC6772561
- DOI: 10.1523/JNEUROSCI.20-15-05775.2000
BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway
Abstract
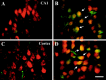
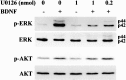
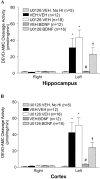
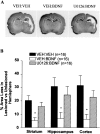
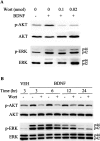
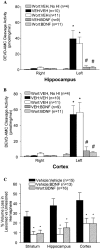
Neurotrophins activate several different intracellular signaling pathways that in some way exert neuroprotective effects. In vitro studies of sympathetic and cerebellar granule neurons have demonstrated that the survival effects of neurotrophins can be mediated via activation of the phosphatidylinositol 3-kinase (PI3-kinase) pathway. Neurotrophin-mediated protection of other neuronal types in vitro can be mediated via the extracellular signal-related protein kinase (ERK) pathway. Whether either of these pathways contributes to the neuroprotective effects of neurotrophins in the brain in vivo has not been determined. Brain-derived neurotrophic factor (BDNF) is markedly neuroprotective against neonatal hypoxic-ischemic (H-I) brain injury in vivo. We assessed the role of the ERK and PI3-kinase pathways in neonatal H-I brain injury in the presence and absence of BDNF. Intracerebroventricular administration of BDNF to postnatal day 7 rats resulted in phosphorylation of ERK1/2 and the PI3-kinase substrate AKT within minutes. Effects were greater on ERK activation and occurred in neurons. Pharmacological inhibition of ERK, but not the PI3-kinase pathway, inhibited the ability of BDNF to block H-I-induced caspase-3 activation and tissue loss. These findings suggest that neuronal ERK activation in the neonatal brain mediates neuroprotection against H-I brain injury, a model of cerebral palsy.
Figures

References
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1365. - PubMed
-
- Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
