Long-lasting inhibitory synaptic depression is age- and calcium-dependent
- PMID: 10908623
- PMCID: PMC6772535
- DOI: 10.1523/JNEUROSCI.20-15-05820.2000
Long-lasting inhibitory synaptic depression is age- and calcium-dependent
Abstract
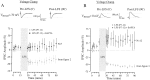
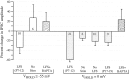
The developmental refinement of excitatory synapses is often influenced by neuronal activity, and underlying synaptic mechanisms have been suggested. In contrast, few studies have asked whether inhibitory synapses are reorganized during development and whether this is accompanied by use-dependent changes of inhibitory synaptic strength. The topographic inhibitory projection from the medial nucleus of the trapezoid body (MNTB) to the lateral superior olive (LSO) undergoes synapse elimination during development (Sanes and Takács, 1993). To determine whether there is an associated period of synaptic plasticity, whole-cell recordings were obtained from developing LSO neurons of gerbils in a brain slice preparation. In current-clamp recordings, low-frequency stimulation of the MNTB led to a decline in IPSP amplitude by 43%. In voltage-clamp recordings, hyperpolarized LSO neurons also exhibited a long-lasting depression of MNTB-evoked inhibitory synaptic currents (34%) after low-frequency stimulation. When LSO neurons were depolarized, low-frequency stimulation of the MNTB produced a significantly larger inhibitory synaptic depression (59%). This synaptic plasticity declined dramatically by postnatal days 17-19. Similar to well studied forms of excitatory synaptic plasticity, inhibitory depression depended on postsynaptic calcium. We propose that such activity-dependent synaptic depression may support the developmental rearrangement of inhibitory terminals as they compete with neighboring excitatory and/or inhibitory inputs.
Figures
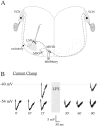
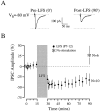
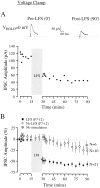
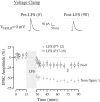
Similar articles
-
Developmental expression of inhibitory synaptic long-term potentiation in the lateral superior olive.Front Neural Circuits. 2014 Jun 19;8:67. doi: 10.3389/fncir.2014.00067. eCollection 2014. Front Neural Circuits. 2014. PMID: 24994969 Free PMC article.
-
Postsynaptic kinase signaling underlies inhibitory synaptic plasticity in the lateral superior olive.J Neurobiol. 2002 Oct;53(1):36-43. doi: 10.1002/neu.10107. J Neurobiol. 2002. PMID: 12360581
-
Deafferentation weakens excitatory synapses in the developing central auditory system.Eur J Neurosci. 1997 Nov;9(11):2340-7. doi: 10.1111/j.1460-9568.1997.tb01651.x. Eur J Neurosci. 1997. PMID: 9464928
-
The many forms and functions of long term plasticity at GABAergic synapses.Neural Plast. 2011;2011:254724. doi: 10.1155/2011/254724. Epub 2011 Jul 21. Neural Plast. 2011. PMID: 21789285 Free PMC article. Review.
-
Extracellular Metalloproteinases in the Plasticity of Excitatory and Inhibitory Synapses.Cells. 2021 Aug 11;10(8):2055. doi: 10.3390/cells10082055. Cells. 2021. PMID: 34440823 Free PMC article. Review.
Cited by
-
Developmental hearing loss eliminates long-term potentiation in the auditory cortex.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3550-5. doi: 10.1073/pnas.0607177104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360680 Free PMC article.
-
Long-term inhibitory plasticity in visual cortical layer 4 switches sign at the opening of the critical period.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4540-7. doi: 10.1073/pnas.1319571110. Epub 2013 Nov 4. Proc Natl Acad Sci U S A. 2013. PMID: 24191045 Free PMC article.
-
Activation of synaptic group II metabotropic glutamate receptors induces long-term depression at GABAergic synapses in CNS neurons.J Neurosci. 2013 Oct 2;33(40):15964-77. doi: 10.1523/JNEUROSCI.0202-13.2013. J Neurosci. 2013. PMID: 24089501 Free PMC article.
-
Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing.Future Neurol. 2009 May 1;4(3):331-349. doi: 10.2217/FNL.09.5. Future Neurol. 2009. PMID: 20161214 Free PMC article.
-
The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain.Mol Pain. 2019 Jan-Dec;15:1744806919847366. doi: 10.1177/1744806919847366. Mol Pain. 2019. PMID: 30977423 Free PMC article. Review.
References
-
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. - PubMed
-
- Battistin T, Cherubini E. Developmental shift from long-term potentiation at the mossy fibre synapses in the rat hippocampus. Eur J Neurosci. 1994;6:1750–1755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
