Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti
- PMID: 10908672
- PMCID: PMC16836
- DOI: 10.1073/pnas.160258197
Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti
Abstract
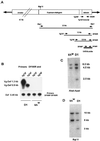
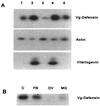
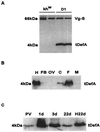
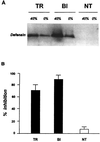
Progress in molecular genetics makes possible the development of alternative disease control strategies that target the competence of mosquitoes to transmit pathogens. We tested the regulatory region of the vitellogenin (Vg) gene of Aedes aegypti for its ability to express potential antipathogen factors in transgenic mosquitoes. Hermes-mediated transformation was used to integrate a 2.1-kb Vg-promoter fragment driving the expression of the Defensin A (DefA) coding region, one of the major insect immune factors. PCR amplification of genomic DNA and Southern blot analyses, carried out through the ninth generation, showed that the Vg-DefA transgene insertion was stable. The Vg-DefA transgene was strongly activated in the fat body by a blood meal. The mRNA levels reached a maximum at 24-h postblood meal, corresponding to the peak expression time of the endogenous Vg gene. High levels of transgenic defensin were accumulated in the hemolymph of bloodfed female mosquitoes, persisting for 20-22 days after a single blood feeding. Purified transgenic defensin showed antibacterial activity comparable to that of defensin isolated from bacterially challenged control mosquitoes. Thus, we have been able to engineer the genetically stable transgenic mosquito with an element of systemic immunity, which is activated through the blood meal-triggered cascade rather than by infection. This work represents a significant step toward the development of molecular genetic approaches to the control of vector competence in pathogen transmission.
Figures
Comment in
-
Genetic manipulation of vectors: A potential novel approach for control of vector-borne diseases.Proc Natl Acad Sci U S A. 2000 Sep 12;97(19):10295-7. doi: 10.1073/pnas.97.19.10295. Proc Natl Acad Sci U S A. 2000. PMID: 10984525 Free PMC article. No abstract available.
References
-
- Butler D. Nature (London) 1997;386:535–536. - PubMed
-
- Bruno J M, Feachem R, Godal T, Nchinda T, Ogilvie B, Mons B, Mshana R, Radda G, Samba E, Schwartz M, et al. Nature (London) 1997;386:541. - PubMed
-
- Beier J C. Annu Rev Entomol. 1998;43:519–543. - PubMed
-
- Collins F H, James A A. Sci Med. 1996;3:52–61.
-
- O'Brochta D A, Atkinson P W. Insect Biochem Mol Biol. 1996;26:739–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

