Protein-interaction modules that organize nuclear function: FF domains of CA150 bind the phosphoCTD of RNA polymerase II
- PMID: 10908677
- PMCID: PMC16813
- DOI: 10.1073/pnas.160266597
Protein-interaction modules that organize nuclear function: FF domains of CA150 bind the phosphoCTD of RNA polymerase II
Abstract
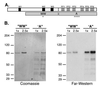
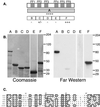
An approach for purifying nuclear proteins that bind directly to the hyperphosphorylated C-terminal repeat domain (CTD) of RNA polymerase II was developed and used to identify one human phosphoCTD-associating protein as CA150. CA150 is a nuclear factor implicated in transcription elongation. Because the hyperphosphorylated CTD is a feature of actively transcribing RNA polymerase II (Pol II), phosphoCTD (PCTD) binding places CA150 in a location appropriate for performing a role in transcription elongation-related events. Several recombinant segments of CA150 bound the PCTD. Predominant binding is mediated by the portion of CA150 containing six FF domains, compact modules of previously unknown function. In fact, small recombinant proteins containing the fifth FF domain bound the PCTD. PCTD binding is the first specific function assigned to an FF domain. As FF domains are found in a variety of nuclear proteins, it is likely that some of these proteins are also PCTD-associating proteins. Thus FF domains appear to be compact protein-interaction modules that, like WW domains, can be evolutionarily shuffled to organize nuclear function.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

