The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon
- PMID: 10913080
- PMCID: PMC94618
- DOI: 10.1128/JB.182.16.4466-4477.2000
The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon
Abstract
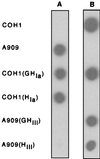
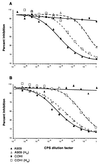
Streptococcus agalactiae is a primary cause of neonatal morbidity and mortality. Essential to the virulence of this pathogen is the production of a type-specific capsular polysaccharide (CPS) that enables the bacteria to evade host immune defenses. The identification, cloning, sequencing, and functional characterization of seven genes involved in type III capsule production have been previously reported. Here, we describe the cloning and sequencing of nine additional adjacent genes, cps(III)FGHIJKL, neu(III)B, and neu(III)C. Sequence comparisons suggested that these genes are involved in sialic acid synthesis, pentasaccharide repeating unit formation, and oligosaccharide transport and polymerization. The type III CPS (cpsIII) locus was comprised of 16 genes within 15.5 kb of contiguous chromosomal DNA. Primer extension analysis and investigation of mRNA from mutants with polar insertions in their cpsIII loci supported the hypothesis that the operon is transcribed as a single polycistronic message. The translated cpsIII sequences were compared to those of the S. agalactiae cpsIa locus, and the primary difference between the operons was found to reside in cps(III)H, the putative CPS polymerase gene. Expression of cps(III)H in a type Ia strain resulted in suppression of CPS Ia synthesis and in production of a CPS which reacted with type III-specific polyclonal antibody. Likewise, expression of the putative type Ia polymerase gene in a type III strain reduced synthesis of type III CPS with production of a type Ia immunoreactive capsule. Based on the similar structures of the oligosaccharide repeating units of the type Ia and III capsules, our observations demonstrated that cps(Ia)H and cps(III)H encoded the type Ia and III CPS polymerases, respectively. Additionally, these findings suggested that a single gene can confer serotype specificity in organisms that produce complex polysaccharides.
Figures
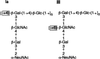




References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons; 1995.
-
- Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders Co.; 1995. pp. 980–1054.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

