A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities
- PMID: 10913089
- PMCID: PMC94627
- DOI: 10.1128/JB.182.16.4557-4563.2000
A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities
Abstract
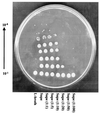
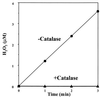
A Pseudomonas aeruginosa oxyR mutant was dramatically sensitive to H(2)O(2), despite possessing wild-type catalase activity. Oxygen-dependent oxyR phenotypes also included an inability to survive aerobic serial dilution in Luria broth and to resist aminoglycosides. Plating the oxyR mutant after serial dilution in its own spent culture supernatant, which contained the major catalase KatA, or under anaerobic conditions allowed for survival. KatA was resistant to sodium dodecyl sulfate, proteinase K, pepsin, trypsin, chymotrypsin and the neutrophil protease cathepsin G. When provided in trans and expressed constitutively, the OxyR-regulated genes katB, ahpB, and ahpCF could not restore both the serial dilution defect and H(2)O(2) resistance; only oxyR itself could do so. The aerobic dilution defect could be complemented, in part, by only ahpB and ahpCF, suggesting that the latter gene products could possess a catalase-like activity. Aerobic Luria broth was found to generate approximately 1.2 microM H(2)O(2) min(-1) via autoxidation, a level sufficient to kill serially diluted oxyR and oxyR katA bacteria and explain the molecular mechanism behind the aerobic serial dilution defect. Taken together, our results indicate that inactivation of OxyR renders P. aeruginosa exquisitely sensitive to both H(2)O(2) and aminoglycosides, which are clinically and environmentally important antimicrobials.
Figures


References
-
- Altuvia S, Almirón M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςS in stationary phase. Mol Microbiol. 1994;13:265–272. - PubMed
-
- Beers R F, Jr, Sizer I W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brown S M, Howell M L, Vasil M L, Anderson A J, Hassett D J. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol. 1995;177:6536–6544. - PMC - PubMed
-
- Christman M F, Morgan R, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

