Vibrio fischeri lux genes play an important role in colonization and development of the host light organ
- PMID: 10913092
- PMCID: PMC94630
- DOI: 10.1128/JB.182.16.4578-4586.2000
Vibrio fischeri lux genes play an important role in colonization and development of the host light organ
Abstract
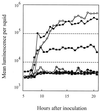
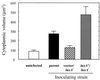
The bioluminescent bacterium Vibrio fischeri and juveniles of the squid Euprymna scolopes specifically recognize and respond to one another during the formation of a persistent colonization within the host's nascent light-emitting organ. The resulting fully developed light organ contains brightly luminescing bacteria and has undergone a bacterium-induced program of tissue differentiation, one component of which is a swelling of the epithelial cells that line the symbiont-containing crypts. While the luminescence (lux) genes of symbiotic V. fischeri have been shown to be highly induced within the crypts, the role of these genes in the initiation and persistence of the symbiosis has not been rigorously examined. We have constructed and examined three mutants (luxA, luxI, and luxR), defective in either luciferase enzymatic or regulatory proteins. All three are unable to induce normal luminescence levels in the host and, 2 days after initiating the association, had a three- to fourfold defect in the extent of colonization. Surprisingly, these lux mutants also were unable to induce swelling in the crypt epithelial cells. Complementing, in trans, the defect in light emission restored both normal colonization capability and induction of swelling. We hypothesize that a diminished level of oxygen consumption by a luciferase-deficient symbiotic population is responsible for the reduced fitness of lux mutants in the light organ crypts. This study is the first to show that the capacity for bioluminescence is critical for normal cell-cell interactions between a bacterium and its animal host and presents the first examples of V. fischeri genes that affect normal host tissue development.
Figures




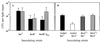
References
-
- Boettcher K J, Ruby E G, McFall-Ngai M J. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179:65–73.
-
- Chatterjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Inactivation of rmsA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol. 1995;61:1959–1967. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

