Cks1 is required for G(1) cyclin-cyclin-dependent kinase activity in budding yeast
- PMID: 10913169
- PMCID: PMC86063
- DOI: 10.1128/MCB.20.16.5858-5864.2000
Cks1 is required for G(1) cyclin-cyclin-dependent kinase activity in budding yeast
Abstract
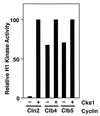
p13(suc1) (Cks) proteins have been implicated in the regulation of cyclin-dependent kinase (CDK) activity. However, the mechanism by which Cks influences the function of cyclin-CDK complexes has remained elusive. We show here that Cks1 is required for the protein kinase activity of budding yeast G(1) cyclin-CDK complexes. Cln2 and Cdc28 subunits coexpressed in baculovirus-infected insect cells fail to exhibit protein kinase activity towards multiple substrates in the absence of Cks1. Cks1 can both stabilize Cln2-Cdc28 complexes and activate intact complexes in vitro, suggesting that it plays multiple roles in the biogenesis of active G(1) cyclin-CDK complexes. In contrast, Cdc28 forms stable, active complexes with the B-type cyclins Clb4 and Clb5 regardless of whether Cks1 is present. The levels of Cln2-Cdc28 and Cln3-Cdc28 protein kinase activity are severely reduced in cks1-38 cell extracts. Moreover, phosphorylation of G(1) cyclins, which depends on Cdc28 activity, is reduced in cks1-38 cells. The role of Cks1 in promoting G(1) cyclin-CDK protein kinase activity both in vitro and in vivo provides a simple molecular rationale for the essential role of CKS1 in progression through G(1) phase in budding yeast.
Figures
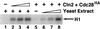
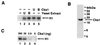




Similar articles
-
Cyclin-dependent kinase and Cks/Suc1 interact with the proteasome in yeast to control proteolysis of M-phase targets.Genes Dev. 1999 May 1;13(9):1190-202. doi: 10.1101/gad.13.9.1190. Genes Dev. 1999. PMID: 10323869 Free PMC article.
-
Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85.Science. 1994 Nov 25;266(5189):1388-91. doi: 10.1126/science.7973730. Science. 1994. PMID: 7973730
-
Interactions between Pho85 cyclin-dependent kinase complexes and the Swi5 transcription factor in budding yeast.Mol Microbiol. 2000 Feb;35(4):825-34. doi: 10.1046/j.1365-2958.2000.01754.x. Mol Microbiol. 2000. PMID: 10692159
-
The cyclin family of budding yeast: abundant use of a good idea.Trends Genet. 1998 Feb;14(2):66-72. doi: 10.1016/s0168-9525(97)01322-x. Trends Genet. 1998. PMID: 9520600 Review.
-
Pho85, a multifunctional cyclin-dependent protein kinase in budding yeast.Mol Microbiol. 2007 Oct;66(2):303-14. doi: 10.1111/j.1365-2958.2007.05914.x. Epub 2007 Sep 10. Mol Microbiol. 2007. PMID: 17850263 Review.
Cited by
-
Cyclin-Dependent Kinase Regulatory Subunit 2 Indicated Poor Prognosis and Facilitated Aggressive Phenotype of Hepatocellular Carcinoma.Dis Markers. 2019 Oct 22;2019:8964015. doi: 10.1155/2019/8964015. eCollection 2019. Dis Markers. 2019. PMID: 31781310 Free PMC article.
-
An overview of Cdk1-controlled targets and processes.Cell Div. 2010 May 13;5:11. doi: 10.1186/1747-1028-5-11. Cell Div. 2010. PMID: 20465793 Free PMC article.
-
MicroRNA-936 induces cell cycle arrest and inhibits glioma cell proliferation by targeting CKS1.Am J Cancer Res. 2017 Nov 1;7(11):2131-2143. eCollection 2017. Am J Cancer Res. 2017. PMID: 29218238 Free PMC article.
-
Phosphate-binding pocket on cyclin B governs CDK substrate phosphorylation and mitotic timing.Nat Commun. 2025 May 8;16(1):4281. doi: 10.1038/s41467-025-59700-7. Nat Commun. 2025. PMID: 40341598 Free PMC article.
-
Expression of CKS2 in Hepatocellular Carcinoma: Correlation with Survival Outcomes and Immune Microenvironment.J Hepatocell Carcinoma. 2023 Oct 9;10:1767-1784. doi: 10.2147/JHC.S427624. eCollection 2023. J Hepatocell Carcinoma. 2023. PMID: 37841370 Free PMC article.
References
-
- Bourne Y, Watson M H, Hickey M J, Holmes W, Rocque W, Reed S I, Tainer J A. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell. 1996;84:863–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
