Regulation of c-SRC activity and function by the adapter protein CAS
- PMID: 10913170
- PMCID: PMC86064
- DOI: 10.1128/MCB.20.16.5865-5878.2000
Regulation of c-SRC activity and function by the adapter protein CAS
Abstract
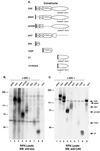
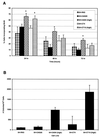
SRC family kinases play essential roles in a variety of cellular functions, including proliferation, survival, differentiation, and apoptosis. The activities of these kinases are regulated by intramolecular interactions and by heterologous binding partners that modulate the transition between active and inactive structural conformations. p130(CAS) (CAS) binds directly to both the SH2 and SH3 domains of c-SRC and therefore has the potential to structurally alter and activate this kinase. In this report, we demonstrate that overexpression of full-length CAS in COS-1 cells induces c-SRC-dependent tyrosine phosphorylation of multiple endogenous cellular proteins. A carboxy-terminal fragment of CAS (CAS-CT), which contains the c-SRC binding site, was sufficient to induce c-SRC-dependent protein tyrosine kinase activity, as measured by tyrosine phosphorylation of cortactin, paxillin, and, to a lesser extent, focal adhesion kinase. A single amino acid substitution located in the binding site for the SRC SH3 domain of CAS-CT disrupted CAS-CT's interaction with c-SRC and inhibited its ability to induce tyrosine phosphorylation of cortactin and paxillin. Murine C3H10T1/2 fibroblasts that expressed elevated levels of tyrosine phosphorylated CAS and c-SRC-CAS complexes exhibited an enhanced ability to form colonies in soft agar and to proliferate in the absence of serum or growth factors. CAS-CT fully substituted for CAS in mediating growth in soft agar but was less effective in promoting serum-independent growth. These data suggest that CAS plays an important role in regulating specific signaling pathways governing cell growth and/or survival, in part through its ability to interact with and modulate the activity of c-SRC.
Figures


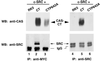

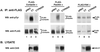

References
-
- Abram C L, Courtneidge S A. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. - PubMed
-
- Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel, p130CAS-related protein, Sin. Genes Dev. 1996;10:1341–1355. - PubMed
-
- Anderson R G W. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Biscardi J S, Belsches A P, Parsons S J. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. - PubMed
-
- Biscardi J S, Tice D A, Parsons S J. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
