TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor
- PMID: 10913174
- PMCID: PMC86068
- DOI: 10.1128/MCB.20.16.5908-5916.2000
TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor
Abstract
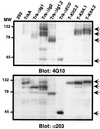
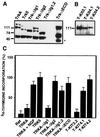
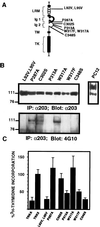
The extracellular region of the nerve growth factor (NGF) receptor, TrkA, contains two immunoglobulin (Ig)-like domains that are required for specific ligand binding. We have investigated the possible role of these two Ig-like domains in receptor dimerization and activation by using different mutants of the TrkA extracellular region. Deletions of each Ig-like domain, of both, and of the entire extracellular region were made. To probe the structural constraints on ligand-independent receptor dimerization, chimeric receptors were generated by swapping the Ig-like domains of the TrkA receptor for the third or fourth Ig-like domain of c-Kit. We also introduced single-amino-acid changes in conserved residues within the Ig-like domains of TrkA. Most of these TrkA variants did not bind NGF, and their expression in PC12nnr5 cells, which lack endogenous TrkA, promoted ligand-independent neurite outgrowth. Some TrkA mutant receptors induced malignant transformation of Rat-1 cells, as assessed by measuring proliferation in the absence of serum, anchorage-independent growth, and tumorigenesis in nude mice. These mutants exhibited constitutive phosphorylation and spontaneous dimerization consistent with their biological activities. Our data suggest that spontaneous dimerization of TrkA occurs when the structure of the Ig-like domains is altered, implying that the intact domains inhibit receptor dimerization in the absence of NGF.
Figures




References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1995.
-
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. - PubMed
-
- Blechman J M, Lev S, Barg J, Eisenstein M, Vaks B, Vogel Z, Givol D, Yarden Y. The fourth immunoglobulin domain of the stem cell factor receptor couples ligand binding to signal transduction. Cell. 1995;80:103–113. - PubMed
-
- Brodeur G M, Maris J M, Yamashiro D J, Hogarty M D, White P S. Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol. 1997;19:93–101. - PubMed
-
- Canossa M, Rovelli G, Shooter E M. Transphosphorylation of the neurotrophin Trk receptors. J Biol Chem. 1996;271:5812–5818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
