Receptor isoforms mediate opposing proliferative effects through gbetagamma-activated p38 or Akt pathways
- PMID: 10913180
- PMCID: PMC86074
- DOI: 10.1128/MCB.20.16.5974-5985.2000
Receptor isoforms mediate opposing proliferative effects through gbetagamma-activated p38 or Akt pathways
Abstract
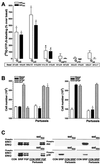
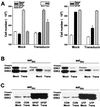
The opposing effects on proliferation mediated by G-protein-coupled receptor isoforms differing in their COOH termini could be correlated with the abilities of the receptors to differentially activate p38, implicated in apoptotic events, or phosphatidylinositol 3-kinase (PI 3-K), which provides a source of survival signals. These contrasting growth responses of the somatostatin sst(2) receptor isoforms, which couple to identical Galpha subunit pools (Galpha(i3) > Galpha(i2) >> Galpha(0)), were both inhibited following betagamma sequestration. The sst(2(a)) receptor-mediated ATF-2 activation and inhibition of proliferation induced by basic fibroblast growth factor (bFGF) were dependent on prolonged phosphorylation of p38. In contrast, cell proliferation and the associated transient phosphorylation of Akt and p70(rsk) induced by sst(2(b)) receptors were blocked by the PI 3-K inhibitor LY 294002. Stimulation with bFGF alone had no effect on the activity of either p38 or Akt but markedly enhanced p38 phosphorylation mediated by sst(2(a)) receptors, suggesting that a complex interplay exists between the transduction cascades activated by these distinct receptor types. In addition, although all receptors mediated a sustained activation of extracellular signal-regulated kinases (ERK1 and ERK2), induction of the tumor suppressor p21(cip1) was detected only following amplification of ERK and p38 phosphorylation by concomitant bFGF and sst(2(a)) receptor activation. Expression of constitutively active Akt in the presence of a p38 inhibitor enabled a proliferative response to be detected in sst(2(a)) receptor-expressing cells. These findings demonstrate that the duration of activation and a critical balance between the mitogen-activated protein kinase and PI 3-K pathways are important for controlling cell proliferation and that the COOH termini of the sst(2) receptor isoforms may determine the selection of appropriate betagamma-pairings necessary for interaction with distinct kinase cascades.
Figures







Similar articles
-
High-intensity p38 kinase activity is critical for p21(cip1) induction and the antiproliferative function of G(i) protein-coupled receptors.Mol Pharmacol. 2001 May;59(5):1119-28. doi: 10.1124/mol.59.5.1119. Mol Pharmacol. 2001. PMID: 11306695
-
G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Galpha(q) and Galpha(i) signals.Mol Cell Biol. 2000 Sep;20(18):6837-48. doi: 10.1128/MCB.20.18.6837-6848.2000. Mol Cell Biol. 2000. PMID: 10958680 Free PMC article.
-
Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors.Am J Physiol Gastrointest Liver Physiol. 2003 Mar;284(3):G472-80. doi: 10.1152/ajpgi.00345.2002. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12576304
-
Multiple pathways of ERK activation by G protein-coupled receptors.Novartis Found Symp. 2001;239:68-79; discussion 80-4, 150-9. doi: 10.1002/0470846674.ch7. Novartis Found Symp. 2001. PMID: 11529317 Review.
-
Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades.Biochem Pharmacol. 1998 Aug 1;56(3):269-77. doi: 10.1016/s0006-2952(98)00059-8. Biochem Pharmacol. 1998. PMID: 9744561 Review.
Cited by
-
Targeting GPCRs and Their Signaling as a Therapeutic Option in Melanoma.Cancers (Basel). 2022 Jan 29;14(3):706. doi: 10.3390/cancers14030706. Cancers (Basel). 2022. PMID: 35158973 Free PMC article. Review.
-
Role of somatostatin receptor-2 in gentamicin-induced auditory hair cell loss in the Mammalian inner ear.PLoS One. 2014 Sep 30;9(9):e108146. doi: 10.1371/journal.pone.0108146. eCollection 2014. PLoS One. 2014. PMID: 25268135 Free PMC article.
-
Somatostatin and Somatostatin Receptors in Tumour Biology.Int J Mol Sci. 2023 Dec 28;25(1):436. doi: 10.3390/ijms25010436. Int J Mol Sci. 2023. PMID: 38203605 Free PMC article. Review.
-
PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms.Theranostics. 2014 Jan 29;4(4):336-65. doi: 10.7150/thno.7851. eCollection 2014. Theranostics. 2014. PMID: 24578720 Free PMC article. Review.
-
Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression.J Mol Signal. 2007 Feb 26;2:2. doi: 10.1186/1750-2187-2-2. J Mol Signal. 2007. PMID: 17319972 Free PMC article.
References
-
- Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Brevini T A L, Bianchi R, Motta M. Direct inhibitory effect of somatostatin on the growth of the human prostatic cancer cell line LNCaP: possible mechanism of action. J Clin Endocrinol Metab. 1993;77:626–631. - PubMed
-
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
