Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant
- PMID: 10913182
- PMCID: PMC86076
- DOI: 10.1128/MCB.20.16.5998-6007.2000
Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant
Abstract
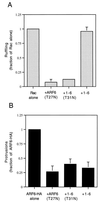
The ADP-ribosylation factor 6 (ARF6) GTPase has a dual function in cells, regulating membrane traffic and organizing cortical actin. ARF6 activation is required for recycling of the endosomal membrane back to the plasma membrane (PM) and also for ruffling at the PM induced by Rac. Additionally, ARF6 at the PM induces the formation of actin-containing protrusions. To identify sequences in ARF6 that are necessary for these distinct functions, we examined the behavior of a chimeric protein of ARF1 and ARF6. The 1-6 chimera (with the amino half of ARF1 and the carboxyl half of ARF6) localized like ARF6 in HeLa cells and moved between the endosome and PM, but it did not form protrusions, an ARF6 effector function. Two residues in the amino-terminal half of ARF6, Q37 and S38, when substituted into the 1-6 chimera allowed protrusion formation, whereas removal of these residues from ARF6 resulted in an inability to form protrusions. Interestingly, expression of 1-6 in cells selectively inhibited protrusions induced by wild-type ARF6 but had no effect on ARF6-regulated membrane movement or Rac-induced ruffling. Thus, we have uncoupled two functions of ARF6, one involved in membrane trafficking, which is necessary for Rac ruffling, and another involved in protrusion formation.
Figures








References
-
- Amor J C, Harrison D H, Kahn R A, Ringe D. Structure of the human ADP ribosylation factor 1 complexed with GDP. Nature. 1994;372:704–708. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1995.
-
- Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
-
- Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. ADP ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
