Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis
- PMID: 10913183
- PMCID: PMC86077
- DOI: 10.1128/MCB.20.16.6008-6018.2000
Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis
Abstract
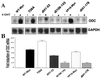
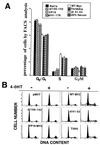
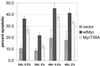
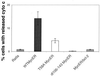
Analysis of amino-terminus mutants of c-Myc has allowed a systematic study of the interrelationship between Myc's ability to regulate transcription and its apoptotic, proliferative, and transforming functions. First, we have found that c-Myc-accelerated apoptosis does not directly correlate with its ability to transactivate transcription using the endogenous ornithine decarboxylase (ODC) gene as readout for transactivation. Furthermore, deletion of the conserved c-Myc box I domain implicated in transactivation does not inhibit apoptosis. Second, the ability of c-Myc to repress transcription, using the gadd45 gene as a readout, correlates with its ability to accelerate apoptosis. A conserved region of c-Myc implicated in mediating transrepression is absolutely required for c-Myc-accelerated apoptosis. Third, a lymphoma-derived Thr58Ala mutation diminishes c-Myc-accelerated apoptosis through a decreased ability to induce the release of cytochrome c from mitochondria. This mutation in a potential phosphorylation site does not affect cell cycle progression, providing genetic evidence that induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc. Finally, we show that the increased ability of Thr58Ala mutant to elicit cellular transformation correlates with its diminished ability to accelerate apoptosis. Bcl-2 overexpression blocked and the lymphoma-associated Thr58Ala mutation decreased c-Myc-accelerated apoptosis, and both led to a significant increase in the ability of Rat1a cells to form colonies in soft agar. This enhanced transformation was greater in soft agar containing a low concentration of serum, suggesting that protection from apoptosis is a mechanism contributing to the increased ability of these cells to proliferate in suspension. Thus, we show here for the first time that, in addition to mutations in complementary antiapoptotic genes, c-Myc itself can acquire mutations that potentiate neoplastic transformation by affecting apoptosis independently of cell cycle progression.
Figures




Similar articles
-
Functional analysis of the N-terminal domain of the Myc oncoprotein.Oncogene. 2003 Apr 3;22(13):1998-2010. doi: 10.1038/sj.onc.1206228. Oncogene. 2003. PMID: 12673205
-
The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals.Mol Cell Biol. 2000 Jun;20(12):4309-19. doi: 10.1128/MCB.20.12.4309-4319.2000. Mol Cell Biol. 2000. PMID: 10825194 Free PMC article.
-
Induction of ornithine decarboxylase by IL-3 is mediated by sequential c-Myc-independent and c-Myc-dependent pathways.Oncogene. 1997 Sep 4;15(10):1219-32. doi: 10.1038/sj.onc.1201273. Oncogene. 1997. PMID: 9294616
-
Integrated control of cell proliferation and cell death by the c-myc oncogene.Philos Trans R Soc Lond B Biol Sci. 1994 Aug 30;345(1313):269-75. doi: 10.1098/rstb.1994.0105. Philos Trans R Soc Lond B Biol Sci. 1994. PMID: 7846125 Review.
-
The functions of Myc in cell cycle progression and apoptosis.Prog Cell Cycle Res. 1996;2:73-82. doi: 10.1007/978-1-4615-5873-6_7. Prog Cell Cycle Res. 1996. PMID: 9552384 Review.
Cited by
-
ETV1 positively regulates transcription of tumor suppressor ARF.Cancer Biol Ther. 2013 Dec;14(12):1167-73. doi: 10.4161/cbt.26883. Epub 2013 Oct 23. Cancer Biol Ther. 2013. PMID: 24157551 Free PMC article.
-
MYC Oncogene Contributions to Release of Cell Cycle Brakes.Genes (Basel). 2019 Mar 22;10(3):244. doi: 10.3390/genes10030244. Genes (Basel). 2019. PMID: 30909496 Free PMC article. Review.
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies.J Hematol Oncol. 2021 Aug 9;14(1):121. doi: 10.1186/s13045-021-01111-4. J Hematol Oncol. 2021. PMID: 34372899 Free PMC article. Review.
-
p53-independent role of MYC mutant T58A in the proliferation and apoptosis of breast cancer cells.Oncol Lett. 2019 Jan;17(1):1071-1079. doi: 10.3892/ol.2018.9688. Epub 2018 Nov 12. Oncol Lett. 2019. PMID: 30655867 Free PMC article.
-
Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis.Mol Cell Biol. 2001 Aug;21(15):5063-70. doi: 10.1128/MCB.21.15.5063-5070.2001. Mol Cell Biol. 2001. PMID: 11438662 Free PMC article.
References
-
- Albert T, Urlbauer B, Kohlhubr F, Hammersen B, Eick D. Ongoing mutations in the N-terminal domain of c-Myc affect transactivation in Burkitt's lymphoma cell lines. Oncogene. 1994;9:759–763. - PubMed
-
- Amati B, Dalton S, Brooks M W, Littlewood T D, Evan G I, Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992;359:423–426. - PubMed
-
- Antonson P, Pray M G, Jacobsson A, Xanthopoulos K G. Myc inhibits CCAAT/enhancer-binding protein alpha-gene expression in HIB-1B hibernoma cells through interactions with the core promoter region. Eur J Biochem. 1995;232:397–403. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
