Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability
- PMID: 10913193
- PMCID: PMC86087
- DOI: 10.1128/MCB.20.16.6114-6126.2000
Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability
Abstract
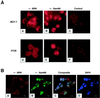
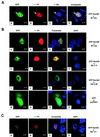
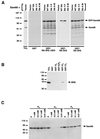
Sik (mouse Src-related intestinal kinase) and its orthologue BRK (human breast tumor kinase) are intracellular tyrosine kinases that are distantly related to the Src family and have a similar structure, but they lack the myristoylation signal. Here we demonstrate that Sik and BRK associate with the RNA binding protein Sam68 (Src associated during mitosis, 68 kDa). We found that Sik interacts with Sam68 through its SH3 and SH2 domains and that the proline-rich P3 region of Sam68 is required for Sik and BRK SH3 binding. In the transformed HT29 adenocarcinoma cell cell line, endogenous BRK and Sam68 colocalize in Sam68-SLM nuclear bodies (SNBs), while transfected Sik and Sam68 are localized diffusely in the nucleoplasm of nontransformed NMuMG mammary epithelial cells. Transfected Sik phosphorylates Sam68 in SNBs in HT29 cells and in the nucleoplasm of NMuMG cells. In functional studies, expression of Sik abolished the ability of Sam68 to bind RNA and act as a cellular Rev homologue. While Sam68 is a substrate for Src family kinases during mitosis, Sik/BRK is the first identified tyrosine kinase that can phosphorylate Sam68 and regulate its activity within the nucleus, where it resides during most of the cell cycle.
Figures
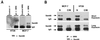




References
-
- Baehrecke E H. who encodes a KH RNA binding protein that functions in muscle development. Development. 1997;124:1323–1332. - PubMed
-
- Barker K T, Jackson L E, Crompton M R. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. - PubMed
-
- Barlat I, Maurier F, Duchesne M, Guitard E, Tocque B, Schweighoffer F. A role for Sam68 in cell cycle progression antagonized by a spliced variant within the KH domain. J Biol Chem. 1997;272:3129–3132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
