BCR-ABL prevents c-jun-mediated and proteasome-dependent FUS (TLS) proteolysis through a protein kinase CbetaII-dependent pathway
- PMID: 10913197
- PMCID: PMC86091
- DOI: 10.1128/MCB.20.16.6159-6169.2000
BCR-ABL prevents c-jun-mediated and proteasome-dependent FUS (TLS) proteolysis through a protein kinase CbetaII-dependent pathway
Abstract
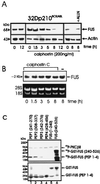
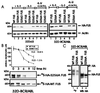
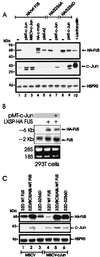
The DNA binding activity of FUS (also known as TLS), a nuclear pro-oncogene involved in multiple translocations, is regulated by BCR-ABL in a protein kinase CbetaII (PKCbetaII)-dependent manner. We show here that in normal myeloid progenitor cells FUS, although not visibly ubiquitinated, undergoes proteasome-dependent degradation, whereas in BCR-ABL-expressing cells, degradation is suppressed by PKCbetaII phosphorylation. Replacement of serine 256 with the phosphomimetic aspartic acid prevents proteasome-dependent proteolysis of FUS, while the serine-256-to-alanine FUS mutant is unstable and susceptible to degradation. Ectopic expression of the phosphomimetic S256D FUS mutant in granulocyte colony-stimulating factor-treated 32Dcl3 cells induces massive apoptosis and inhibits the differentiation of the cells escaping cell death, while the degradation-prone S256A mutant has no effect on either survival or differentiation. FUS proteolysis is induced by c-Jun, is suppressed by BCR-ABL or Jun kinase 1, and does not depend on c-Jun transactivation potential, ubiquitination, or its interaction with Jun kinase 1. In addition, c-Jun-induced FUS proteasome-dependent degradation is enhanced by heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and depends on the formation of a FUS-Jun-hnRNP A1-containing complex and on lack of PKCbetaII phosphorylation at serine 256 but not on FUS ubiquitination. Thus, novel mechanisms appear to be involved in the degradation of FUS in normal myeloid cells; moreover, the ability of the BCR-ABL oncoprotein to suppress FUS degradation by the induction of posttranslational modifications might contribute to the phenotype of BCR-ABL-expressing hematopoietic cells.
Figures





Similar articles
-
hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis.Mol Cell Biol. 2002 Apr;22(7):2255-66. doi: 10.1128/MCB.22.7.2255-2266.2002. Mol Cell Biol. 2002. PMID: 11884611 Free PMC article.
-
TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis.EMBO J. 1998 Aug 3;17(15):4442-55. doi: 10.1093/emboj/17.15.4442. EMBO J. 1998. PMID: 9687511 Free PMC article.
-
Bcr-Abl kinase down-regulates cyclin-dependent kinase inhibitor p27 in human and murine cell lines.Blood. 2000 Sep 1;96(5):1933-9. Blood. 2000. PMID: 10961897
-
Proteasome inhibition leads to significant reduction of Bcr-Abl expression and subsequent induction of apoptosis in K562 human chronic myelogenous leukemia cells.J Pharmacol Exp Ther. 1999 May;289(2):781-90. J Pharmacol Exp Ther. 1999. PMID: 10215653
-
Regulation of the c-jun gene in p210 BCR-ABL transformed cells corresponds with activity of JNK, the c-jun N-terminal kinase.Blood. 1998 Oct 1;92(7):2450-60. Blood. 1998. PMID: 9746785
Cited by
-
NPM/ALK binds and phosphorylates the RNA/DNA-binding protein PSF in anaplastic large-cell lymphoma.Blood. 2007 Oct 1;110(7):2600-9. doi: 10.1182/blood-2006-01-028647. Epub 2007 May 30. Blood. 2007. PMID: 17537995 Free PMC article.
-
Nuclear RNA transcript levels modulate nucleocytoplasmic distribution of ALS/FTD-associated protein FUS.Sci Rep. 2022 May 17;12(1):8180. doi: 10.1038/s41598-022-12098-4. Sci Rep. 2022. PMID: 35581240 Free PMC article.
-
Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations.Nucleic Acids Res. 2011 Apr;39(7):2781-98. doi: 10.1093/nar/gkq1162. Epub 2010 Nov 24. Nucleic Acids Res. 2011. PMID: 21109527 Free PMC article.
-
The oncogenic TLS-ERG fusion protein exerts different effects in hematopoietic cells and fibroblasts.Mol Cell Biol. 2005 Jul;25(14):6235-46. doi: 10.1128/MCB.25.14.6235-6246.2005. Mol Cell Biol. 2005. PMID: 15988032 Free PMC article.
-
The Role of Post-Translational Modifications on Prion-Like Aggregation and Liquid-Phase Separation of FUS.Int J Mol Sci. 2018 Mar 16;19(3):886. doi: 10.3390/ijms19030886. Int J Mol Sci. 2018. PMID: 29547565 Free PMC article. Review.
References
-
- Bercovich Z, Rosenberg-Hasson Y, Ciechanover A, Kahana C. Degradation of ornithine decarboxylase in reticulocyte lysate is ATP-dependent but ubiquitin-independent. J Biol Chem. 1989;264:15949–15952. - PubMed
-
- Bouvet P, Diaz J J, Kindbeiter K, Madjar J J, Amalric F. Nucleolin interacts with several ribosomal proteins through its RGG domain. J Biol Chem. 1998;273:19025–19029. - PubMed
-
- Bureau J P, Henry L, Baz A, Scherrer K, Chateau M T. Prosome (proteasome) changes during differentiation are related to the type of inducer. Mol Biol Rep. 1997;24:57–62. - PubMed
-
- Cartegni L, Maconi M, Morandi E, Cobianchi F, Riva S, Biamonti G. hnRNP A1 selectively interacts through its Gly-rich domain with different RNA-binding proteins. J Mol Biol. 1996;259:337–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
