Delayed oral estradiol combined with leuprolide increases endometriosis-related pain
- PMID: 10917114
- PMCID: PMC3015370
Delayed oral estradiol combined with leuprolide increases endometriosis-related pain
Abstract
Objectives: To determine if low-dose estrogen replacement can be added to GnRH agonist therapy after three months to reduce hypoestrogenic symptoms while allowing continued relief of pain in patients with endometriosis.
Materials and methods: Thirteen women with endometriosis and pain were treated with six months of leuprolide acetate in a prospective, randomized double-blind placebo controlled study. After three months of therapy, six subjects initiated oral estradiol 1 mg daily, and seven received an identical placebo.
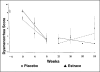
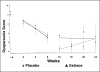
Results: Dysmenorrhea improved in both groups, and dyspareunia significantly improved in the GnRH agonist plus placebo group. The mean pain scores of the oral estrogen group tended to be higher than the placebo group, and hot flushes tended to be less severe with estrogen treatment. However, differences observed between the study and placebo groups did not reach statistical significance.
Conclusion: In a prospective, randomized study, low-dose estrogen replacement increases endometriosis-related pain during GnRH agonist therapy. The study was terminated after the first 13 subjects due to the concerning trend toward recurrent symptoms in women who received oral estradiol during GnRH agonist therapy for endometriosis-related pain. With the trend toward increasing pain with estrogen add-back therapy, a larger study would not seem to be justifiable.
Figures
References
-
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with danazol for endometriosis: a multicenter double-blind comparative trial. N Engl J Med. 1988;318:485–490 - PubMed
-
- Wheeler JM, Knittle JD, Miller JD. Depot leuprolide acetate versus danazol in the treatment of women with symptomatic endometriosis: a multicenter, double-blind randomized clinical trial: I. Efficacy results. Am J Obstet Gynecol. 1992;167:1367–1371 - PubMed
-
- Shaw RW. GnRH analogues in the treatment of endometriosis – rationale and efficacy. In: Thomas EJ, Rock JA, eds. Modern Approaches to Endometriosis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991:257–274
-
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745 - PubMed
-
- Hornstein MD, Surrey ES, Weisberg GW, et al. Lupron add-back study group: leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Obstet Gynecol. 1998;91:16–24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
