Characterization of S-triazine herbicide metabolism by a Nocardioides sp. isolated from agricultural soils
- PMID: 10919761
- PMCID: PMC92125
- DOI: 10.1128/AEM.66.8.3134-3141.2000
Characterization of S-triazine herbicide metabolism by a Nocardioides sp. isolated from agricultural soils
Abstract
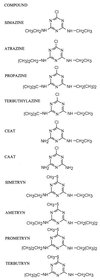
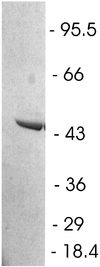
Atrazine, a herbicide widely used in corn production, is a frequently detected groundwater contaminant. Nine gram-positive bacterial strains able to use this herbicide as a sole source of nitrogen were isolated from four farms in central Canada. The strains were divided into two groups based on repetitive extragenic palindromic (rep)-PCR genomic fingerprinting with ERIC and BOXA1R primers. Based on 16S ribosomal DNA sequence analysis, both groups were identified as Nocardioides sp. strains. None of the isolates mineralized [ring-U-(14)C]atrazine. There was no hybridization to genomic DNA from these strains using atzABC cloned from Pseudomonas sp. strain ADP or trzA cloned from Rhodococcus corallinus. S-Triazine degradation was studied in detail in Nocardioides sp. strain C190. Oxygen was not required for atrazine degradation by whole cells or cell extracts. Based on high-pressure liquid chromatography and mass spectrometric analyses of products formed from atrazine in incubations of whole cells with H(2)(18)O, sequential hydrolytic reactions converted atrazine to hydroxyatrazine and then to the end product N-ethylammelide. Isopropylamine, the putative product of the second hydrolytic reaction, supported growth as the sole carbon and nitrogen source. The triazine hydrolase from strain C190 was isolated and purified and found to have a K(m) for atrazine of 25 microM and a V(max) of 31 micromol/min/mg of protein. The subunit molecular mass of the protein was 52 kDa. Atrazine hydrolysis was not inhibited by 500 microM EDTA but was inhibited by 100 microM Mg, Cu, Co, or Zn. Whole cells and purified triazine hydrolase converted a range of chlorine or methylthio-substituted herbicides to the corresponding hydroxy derivatives. In summary, an atrazine-metabolizing Nocardioides sp. widely distributed in agricultural soils degrades a range of s-triazine herbicides by means of a novel s-triazine hydrolase.
Figures




References
-
- Agertved J, Rugge K, Barker J F. Transformation of the herbicides MCPP and atrazine under natural aquifer conditions. Ground Water. 1992;30:500–506.
-
- Alvey S, Crowley D E. Survival and activity of an atrazine-mineralizing bacterial consortium in rhizosphere soil. Environ Sci Technol. 1996;30:1596–1603.
-
- Assaf N A, Turco R F. Accelerated biodegradation of atrazine by a microbial consortium is possible in culture and soil. Biodegradation. 1994;5:29–35. - PubMed
-
- Barriuso E, Houot S. Rapid mineralization of the S-triazine ring of atrazine in soils in relation to soil management. Soil Biol Biochem. 1996;28:1341–1348.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

