Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates
- PMID: 10919779
- PMCID: PMC92143
- DOI: 10.1128/AEM.66.8.3262-3268.2000
Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates
Abstract
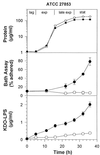
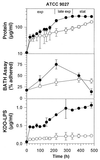
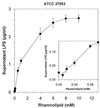
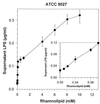
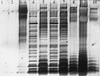
Little is known about the interaction of biosurfactants with bacterial cells. Recent work in the area of biodegradation suggests that there are two mechanisms by which biosurfactants enhance the biodegradation of slightly soluble organic compounds. First, biosurfactants can solubilize hydrophobic compounds within micelle structures, effectively increasing the apparent aqueous solubility of the organic compound and its availability for uptake by a cell. Second, biosurfactants can cause the cell surface to become more hydrophobic, thereby increasing the association of the cell with the slightly soluble substrate. Since the second mechanism requires very low levels of added biosurfactant, it is the more intriguing of the two mechanisms from the perspective of enhancing the biodegradation process. This is because, in practical terms, addition of low levels of biosurfactants will be more cost-effective for bioremediation. To successfully optimize the use of biosurfactants in the bioremediation process, their effect on cell surfaces must be understood. We report here that rhamnolipid biosurfactant causes the cell surface of Pseudomonas spp. to become hydrophobic through release of lipopolysaccharide (LPS). In this study, two Pseudomonas aeruginosa strains were grown on glucose and hexadecane to investigate the chemical and structural changes that occur in the presence of a rhamnolipid biosurfactant. Results showed that rhamnolipids caused an overall loss in cellular fatty acid content. Loss of fatty acids was due to release of LPS from the outer membrane, as demonstrated by 2-keto-3-deoxyoctonic acid and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and further confirmed by scanning electron microscopy. The amount of LPS loss was found to be dependent on rhamnolipid concentration, but significant loss occurred even at concentrations less than the critical micelle concentration. We conclude that rhamnolipid-induced LPS release is the probable mechanism of enhanced cell surface hydrophobicity.
Figures



References
-
- Aronstein B N, Alexander M. Surfactants at low concentrations stimulate biodegradation of sorbed hydrocarbons in samples of aquifer sands and soil slurries. Environ Toxicol Chem. 1992;11:1227–1233.
-
- Churchill S A, Griffin R A, Jones L P, Churchill P F. Biodegradation rate enhancement of hydrocarbons by an oleophilic fertilizer and a rhamnolipid biosurfactant. J Environ Qual. 1995;24:19–28.
-
- Folch J, Lees M, Sloane Stanley G H. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497–509. - PubMed
-
- Gray G W, Wilkinson S G. The action of ethylenediaminetetraacetic acid on Pseudomonas aeruginosa. J Appl Bacteriol. 1965;28:153–164.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

