Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation
- PMID: 10919823
- PMCID: PMC92187
- DOI: 10.1128/AEM.66.8.3574-3585.2000
Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation
Abstract
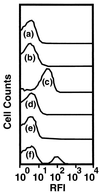
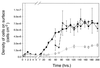
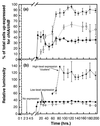
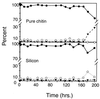
Growth of the chitin-degrading marine bacterium S91 on solid surfaces under oligotrophic conditions was accompanied by the displacement of a large fraction of the surface-derived bacterial production into the flowing bulk aqueous phase, irrespective of the value of the surface as a nutrient source. Over a 200-h period of surface colonization, 97 and 75% of the bacterial biomass generated on biodegradable chitin and a nonnutritional silicon surface, respectively, detached to become part of the free-living population in the bulk aqueous phase. Specific surface-associated growth rates that included the cells that subsequently detached from the substrata varied depending on the nutritional value of the substratum and during the period of surface colonization. Specific growth rates of 3.79 and 2.83 day(-1) were obtained when cells first began to proliferate on a pure chitin film and a silicon surface, respectively. Later, when cell densities on the surface and detached cells as CFU in the bulk aqueous phase achieved a quasi-steady state, specific growth rates decreased to 1.08 and 0.79 day(-1) on the chitin and silicon surfaces, respectively. Virtually all of the cells that detached from either the chitin or the silicon surfaces and the majority of cells associated with the chitin surface over the 200-h period of surface colonization displayed no detectable expression of the chitin-degrading genes chiA and chiB. Cells displaying high levels of chiA-chiB expression were detected only on the chitin surface and then only clustered in discrete areas of the surface. Surface-associated, differential gene expression and displacement of bacterial production from surfaces represent adaptations at the population level that promote efficient utilization of limited resources and dispersal of progeny to maximize access to new sources of energy and maintenance of the population.
Figures




References
-
- Alldredge A L, Gotschalk C. In situ settling behavior of marine snow. Limnol Oceanogr. 1988;33:339–351.
-
- Alldredge A L, Cole J J, Caron D A. Production of heterotrophic bacteria inhabiting macroscopic organic aggregates (marine snow) from surface waters. Limnol Oceanogr. 1986;31:68–78.
-
- Azam F. Microbial control of oceanic carbon flux: the plot thickens. Science. 1998;280:694–696.
-
- Azam F, Hodson R. Size distribution and activity of marine microheterotrophs. Limnol Oceanogr. 1977;22:492–501.
-
- Bae H, Hudson S M. The cooperative binding behavior of sodium dodecyl sulfate to crosslinked chitosan films. J Appl Polymer Sci: Part A Polymer Chem. 1997;35:3755–3765.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

