Transformation of amoxapine by Cunninghamella elegans
- PMID: 10919836
- PMCID: PMC92200
- DOI: 10.1128/AEM.66.8.3646-3649.2000
Transformation of amoxapine by Cunninghamella elegans
Abstract
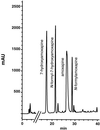
We examined Cunninghamella elegans to determine its ability to transform amoxapine, a tricyclic antidepressant belonging to the dibenzoxazepine class of drugs. Approximately 57% of the exogenous amoxapine was metabolized to three metabolites that were isolated by high-performance liquid chromatography and were identified by nuclear magnetic resonance and mass spectrometry as 7-hydroxyamoxapine (48%), N-formyl-7-hydroxyamoxapine (31%), and N-formylamoxapine (21%). 7-Hydroxyamoxapine, a mammalian metabolite with biological activity, now can be produced in milligram quantities for toxicological evaluation.
Figures
References
-
- Ban T A, Fujimori M, Petrie W M, Ragheb M, Wilson W H. Systematic studies with amoxapine, a new antidepressant. Int Pharmacopsychiatry. 1982;17:18–27. - PubMed
-
- Calvo B, Garcia M J, Pedraz J L, Marino E L, Dominguez-Gil A. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23:180–185. - PubMed
-
- Cerniglia C E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. - PubMed
-
- Cerniglia C E, Gibson D T. Metabolism of naphthalene by cell-extracts of Cunninghamella elegans. Arch Biochem Biophys. 1978;186:121–127. - PubMed
-
- Clark A M, Hufford C D. Use of microorganisms for the study of drug metabolism: an update. Med Res Rev. 1991;11:473–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources