Severed molecules functionally define the boundaries of the cystic fibrosis transmembrane conductance regulator's NH(2)-terminal nucleotide binding domain
- PMID: 10919864
- PMCID: PMC2229491
- DOI: 10.1085/jgp.116.2.163
Severed molecules functionally define the boundaries of the cystic fibrosis transmembrane conductance regulator's NH(2)-terminal nucleotide binding domain
Abstract
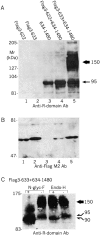
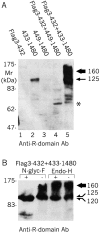
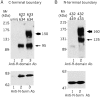
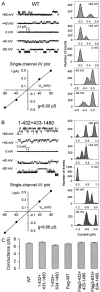
The cystic fibrosis transmembrane conductance regulator is a Cl(-) channel that belongs to the family of ATP-binding cassette proteins. The CFTR polypeptide comprises two transmembrane domains, two nucleotide binding domains (NBD1 and NBD2), and a regulatory (R) domain. Gating of the channel is controlled by kinase-mediated phosphorylation of the R domain and by ATP binding, and, likely, hydrolysis at the NBDs. Exon 13 of the CFTR gene encodes amino acids (aa's) 590-830, which were originally ascribed to the R domain. In this study, CFTR channels were severed near likely NH(2)- or COOH-terminal boundaries of NBD1. CFTR channel activity, assayed using two-microelectrode voltage clamp and excised patch recordings, provided a sensitive measure of successful assembly of each pair of channel segments as the sever point was systematically shifted along the primary sequence. Substantial channel activity was taken as an indication that NBD1 was functionally intact. This approach revealed that the COOH terminus of NBD1 extends beyond aa 590 and lies between aa's 622 and 634, while the NH(2) terminus of NBD1 lies between aa's 432 and 449. To facilitate biochemical studies of the expressed proteins, a Flag epitope was added to the NH(2) termini of full length CFTR, and of CFTR segments truncated before the normal COOH terminus (aa 1480). The functionally identified NBD1 boundaries are supported by Western blotting, coimmunoprecipitation, and deglycosylation studies, which showed that an NH(2)-terminal segment representing aa's 3-622 (Flag3-622) or 3-633 (Flag3-633) could physically associate with a COOH-terminal fragment representing aa's 634-1480 (634-1480); however, the latter fragment was glycosylated to the mature form only in the presence of Flag3-633. Similarly, 433-1480 could physically associate with Flag3-432 and was glycosylated to the mature form; however, 449-1480 protein seemed unstable and could hardly be detected even when expressed with Flag3-432. In excised-patch recordings, all functional severed CFTR channels displayed the hallmark characteristics of CFTR, including the requirement of phosphorylation and exposure to MgATP for gating, ability to be locked open by pyrophosphate or AMP-PNP, small single channel conductances, and high apparent affinity of channel opening by MgATP. Our definitions of the boundaries of the NBD1 domain in CFTR are supported by comparison with the solved NBD structures of HisP and RbsA.
Figures






References
-
- Al-Nakkash L., Hwang T.-C. Activation of wild-type and deltaF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflügers Arch. 1999;437:553–561. - PubMed
-
- Annereau J.-P., Wulbrand U., Vankeerberghen A., Cuppens H., Bontems F., Tümmler B., Cassiman J.-J., Stoven V. A novel model for the first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator. FEBS Lett. 1997;407:303–308. - PubMed
-
- Armstrong S., Tabernero L., Zhang H., Hermodson M., Stauffacher C. The 2.5 Å structure of the N-terminal ATP-binding cassette of the ribose ABC transporter. Biophys. J. 1998;74:A338.
-
- Bear C.E., Duguay F., Naismith A.L., Kartner N., Hanrahan H.W., Riordan J.R. Cl− channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J. Biol. Chem. 1991;266:19142–19145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

