Crystal structures of the metal-dependent 2-dehydro-3-deoxy-galactarate aldolase suggest a novel reaction mechanism
- PMID: 10921867
- PMCID: PMC306599
- DOI: 10.1093/emboj/19.15.3849
Crystal structures of the metal-dependent 2-dehydro-3-deoxy-galactarate aldolase suggest a novel reaction mechanism
Abstract
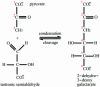
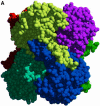
Carbon-carbon bond formation is an essential reaction in organic chemistry and the use of aldolase enzymes for the stereochemical control of such reactions is an attractive alternative to conventional chemical methods. Here we describe the crystal structures of a novel class II enzyme, 2-dehydro-3-deoxy-galactarate (DDG) aldolase from Escherichia coli, in the presence and absence of substrate. The crystal structure was determined by locating only four Se sites to obtain phases for 506 protein residues. The protomer displays a modified (alpha/beta)(8) barrel fold, in which the eighth alpha-helix points away from the beta-barrel instead of packing against it. Analysis of the DDG aldolase crystal structures suggests a novel aldolase mechanism in which a phosphate anion accepts the proton from the methyl group of pyruvate.
Figures
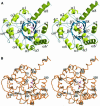





Similar articles
-
Crystal structure and stereochemical studies of KD(P)G aldolase from Thermoproteus tenax.Proteins. 2008 Jul;72(1):35-43. doi: 10.1002/prot.21890. Proteins. 2008. PMID: 18186475
-
Catalytic mechanism of the metal-dependent fuculose aldolase from Escherichia coli as derived from the structure.J Mol Biol. 1996 Jun 14;259(3):458-66. doi: 10.1006/jmbi.1996.0332. J Mol Biol. 1996. PMID: 8676381
-
Analysis of the class I aldolase binding site architecture based on the crystal structure of 2-deoxyribose-5-phosphate aldolase at 0.99A resolution.J Mol Biol. 2004 Oct 29;343(4):1019-34. doi: 10.1016/j.jmb.2004.08.066. J Mol Biol. 2004. PMID: 15476818
-
Rhombohedral crystals of 2-dehydro-3-deoxygalactarate aldolase from Escherichia coli.Acta Crystallogr D Biol Crystallogr. 1999 Jul;55(Pt 7):1368-9. doi: 10.1107/s0907444999006502. Acta Crystallogr D Biol Crystallogr. 1999. PMID: 10393309
-
Purification and biochemical characterization of a pyruvate-specific class II aldolase, HpaI.Biochemistry. 2005 Jul 12;44(27):9447-55. doi: 10.1021/bi050607y. Biochemistry. 2005. PMID: 15996099
Cited by
-
Purification, crystallization and preliminary crystallographic studies on 2-dehydro-3-deoxygalactarate aldolase from Leptospira interrogans.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006 Dec 1;62(Pt 12):1269-70. doi: 10.1107/S174430910604797X. Epub 2006 Nov 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006. PMID: 17142914 Free PMC article.
-
Proteomic analysis of streptomycin resistant and sensitive clinical isolates of Mycobacterium tuberculosis.Proteome Sci. 2010 Nov 18;8:59. doi: 10.1186/1477-5956-8-59. Proteome Sci. 2010. PMID: 21083941 Free PMC article.
-
Identification of a hydratase and a class II aldolase involved in biodegradation of the organic solvent tetralin.Appl Environ Microbiol. 2002 Oct;68(10):4841-6. doi: 10.1128/AEM.68.10.4841-4846.2002. Appl Environ Microbiol. 2002. PMID: 12324329 Free PMC article.
-
Expression, purification and preliminary crystallographic analysis of 2,4-dihydroxy-hepta-2-ene-1,7-dioate aldolase (HpcH) from Escherichia coli C.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Sep 1;61(Pt 9):821-4. doi: 10.1107/S1744309105023079. Epub 2005 Aug 31. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005. PMID: 16511168 Free PMC article.
-
Crystal structure of reaction intermediates in pyruvate class II aldolase: substrate cleavage, enolate stabilization, and substrate specificity.J Biol Chem. 2012 Oct 19;287(43):36208-21. doi: 10.1074/jbc.M112.400705. Epub 2012 Aug 20. J Biol Chem. 2012. PMID: 22908224 Free PMC article.
References
-
- Bacon D.J. and Anderson,W.F. (1988) A fast algorithm for rendering space-filling molecule pictures. J. Mol. Graph., 6, 219–220.
-
- Barbas C.F. III et al. (1997) Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science, 278, 2085–2092. - PubMed
-
- Blackwell N.C. (2000) Mechanistic and structural investigation of DDG aldolase. PhD thesis, University of Leicester, Leicester, UK.
-
- Blackwell N.C., Cullis,P.M., Cooper,R.A. and Izard,T. (1999) Rhombohedral crystals of 2-dehydro-3-deoxy galactarate aldolase from Escherichia coli. Acta Crystallogr. D, 55, 1368–1369. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

