Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell
- PMID: 10921869
- PMCID: PMC306593
- DOI: 10.1093/emboj/19.15.3870
Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell
Abstract
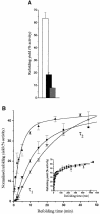
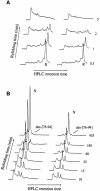
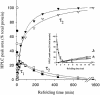
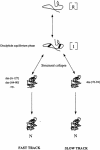
We have studied the effects of macromolecular crowding on protein folding kinetics by studying the oxidative refolding of hen lysozyme in the absence and presence of high concentrations of bovine serum albumin and Ficoll 70. The heterogeneity characteristic of the lysozyme refolding process is preserved under crowded conditions. This, together with the observation that the refolding intermediates that accumulate to significant levels are very similar in the absence and presence of Ficoll, suggests that crowding does not alter substantially the energetics of the protein folding reaction. However, the presence of high concentrations of macromolecules results in the acceleration of the fast track of the refolding process whereas the slow track is substantially retarded. The results can be explained by preferential excluded volume stabilization of compact states relative to more unfolded states, and suggest that, relative to dilute solutions, the rates of many protein folding processes are likely to be altered under conditions that more closely resemble the intracellular environment.
Figures
Similar articles
-
Mixed macromolecular crowding accelerates the oxidative refolding of reduced, denatured lysozyme: implications for protein folding in intracellular environments.J Biol Chem. 2004 Dec 31;279(53):55109-16. doi: 10.1074/jbc.M409086200. Epub 2004 Oct 19. J Biol Chem. 2004. PMID: 15494409
-
Effects of macromolecular crowding on protein folding and aggregation.EMBO J. 1999 Dec 15;18(24):6927-33. doi: 10.1093/emboj/18.24.6927. EMBO J. 1999. PMID: 10601015 Free PMC article.
-
Effects of macromolecular crowding on the folding and aggregation of glycosylated MUC5AC.Biochem Biophys Res Commun. 2020 Sep 3;529(4):984-990. doi: 10.1016/j.bbrc.2020.06.156. Epub 2020 Jul 30. Biochem Biophys Res Commun. 2020. PMID: 32819609
-
Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations.J Pharm Sci. 2005 Aug;94(8):1668-75. doi: 10.1002/jps.20417. J Pharm Sci. 2005. PMID: 15986476 Review.
-
Macromolecular crowding: Macromolecules friend or foe.Biochim Biophys Acta. 2015 Sep;1850(9):1822-31. doi: 10.1016/j.bbagen.2015.05.002. Epub 2015 May 8. Biochim Biophys Acta. 2015. PMID: 25960386 Review.
Cited by
-
Crowding effects on the small, fast-folding protein lambda6-85.Faraday Discuss. 2012;157:451-62; discussion 475-500. doi: 10.1039/c2fd20009k. Faraday Discuss. 2012. PMID: 23230782 Free PMC article.
-
Unfolding of Green Fluorescent Protein mut2 in wet nanoporous silica gels.Protein Sci. 2005 May;14(5):1125-33. doi: 10.1110/ps.041190805. Epub 2005 Mar 31. Protein Sci. 2005. PMID: 15802645 Free PMC article.
-
Crowded, cell-like environment induces shape changes in aspherical protein.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11754-9. doi: 10.1073/pnas.0803672105. Epub 2008 Aug 12. Proc Natl Acad Sci U S A. 2008. PMID: 18697933 Free PMC article.
-
A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors.Sensors (Basel). 2021 Feb 5;21(4):1109. doi: 10.3390/s21041109. Sensors (Basel). 2021. PMID: 33562639 Free PMC article. Review.
-
The protein kingdom extended: ordered and intrinsically disordered proteins, their folding, supramolecular complex formation, and aggregation.Prog Biophys Mol Biol. 2010 Jun-Jul;102(2-3):73-84. doi: 10.1016/j.pbiomolbio.2010.01.003. Epub 2010 Jan 25. Prog Biophys Mol Biol. 2010. PMID: 20097220 Free PMC article. Review.
References
-
- Baldwin R.L. (1995) The nature of protein folding pathways: the classical versus the new view. J. Biomol. NMR, 5, 103–109. - PubMed
-
- Dill K.A. and Chan,H.S. (1997) From Levinthal to pathways to funnels. Nature Struct. Biol., 4, 10–19. - PubMed
-
- Dobson C.M. and Karplus,M. (1999) The fundamentals of protein folding: bringing together theory and experiment. Curr. Opin. Struct. Biol., 9, 92–101. - PubMed
-
- Dobson C.M., Evans,P.A. and Radford,S.E. (1994) Understanding how proteins fold: the lysozyme story so far. Trends Biochem. Sci., 19, 31–37. - PubMed
-
- Dobson C.M., Sali,A. and Karplus,M. (1998) Protein folding: a perspective from theory and experiment. Angew. Chem. Int. Ed., 37, 868–893. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

