Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell
- PMID: 10921869
- PMCID: PMC306593
- DOI: 10.1093/emboj/19.15.3870
Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell
Abstract
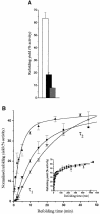
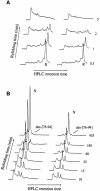
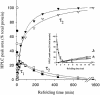
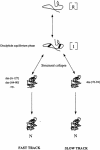
We have studied the effects of macromolecular crowding on protein folding kinetics by studying the oxidative refolding of hen lysozyme in the absence and presence of high concentrations of bovine serum albumin and Ficoll 70. The heterogeneity characteristic of the lysozyme refolding process is preserved under crowded conditions. This, together with the observation that the refolding intermediates that accumulate to significant levels are very similar in the absence and presence of Ficoll, suggests that crowding does not alter substantially the energetics of the protein folding reaction. However, the presence of high concentrations of macromolecules results in the acceleration of the fast track of the refolding process whereas the slow track is substantially retarded. The results can be explained by preferential excluded volume stabilization of compact states relative to more unfolded states, and suggest that, relative to dilute solutions, the rates of many protein folding processes are likely to be altered under conditions that more closely resemble the intracellular environment.
Figures
References
-
- Baldwin R.L. (1995) The nature of protein folding pathways: the classical versus the new view. J. Biomol. NMR, 5, 103–109. - PubMed
-
- Dill K.A. and Chan,H.S. (1997) From Levinthal to pathways to funnels. Nature Struct. Biol., 4, 10–19. - PubMed
-
- Dobson C.M. and Karplus,M. (1999) The fundamentals of protein folding: bringing together theory and experiment. Curr. Opin. Struct. Biol., 9, 92–101. - PubMed
-
- Dobson C.M., Evans,P.A. and Radford,S.E. (1994) Understanding how proteins fold: the lysozyme story so far. Trends Biochem. Sci., 19, 31–37. - PubMed
-
- Dobson C.M., Sali,A. and Karplus,M. (1998) Protein folding: a perspective from theory and experiment. Angew. Chem. Int. Ed., 37, 868–893. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

