Fission yeast Fizzy-related protein srw1p is a G(1)-specific promoter of mitotic cyclin B degradation
- PMID: 10921878
- PMCID: PMC306604
- DOI: 10.1093/emboj/19.15.3968
Fission yeast Fizzy-related protein srw1p is a G(1)-specific promoter of mitotic cyclin B degradation
Abstract
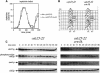
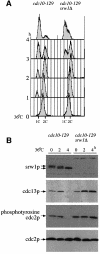
Downregulation of cyclin-dependent kinase (Cdk)-mitotic cyclin complexes is important during cell cycle progression and in G(1) arrested cells undergoing differentiation. srw1p, a member of the Fizzy-related protein family in fission yeast, is required for the degradation of cdc13p mitotic cyclin B during G(1) arrest. Here we show that srw1p is not required for the degradation of cdc13p during mitotic exit demonstrating that there are two systems operative at different stages of the cell cycle for cdc13p degradation, and that srw1p is phosphorylated by Cdk-cdc13p only becoming dephosphorylated during G(1) arrest. We propose that this phosphorylation targets srw1p for proteolysis and inhibits its activity to promote cdc13p turnover.
Figures

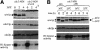
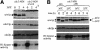





References
-
- Amon A., Irniger,S. and Nasmyth,K. (1994) Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell, 77, 1037–1050. - PubMed
-
- Ayscough K. and Warren,G. (1994) Inhibition of protein synthesis disrupts the Golgi apparatus in the fission yeast, Schizosaccharomyces pombe. Yeast, 10, 1–11. - PubMed
-
- Bähler J., Wu,J.Q., Longtine,M.S., Shah,N.G., McKenzie,A.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

